What is Brucella?
- Brucella is a genus of Gram-negative bacteria.
- It includes several species: B. melitensis, B. abortus, B. suis, and B. canis.
- These bacteria are facultative intracellular pathogens.
- They primarily infect mammals, including livestock and dogs.
- Transmission to humans occurs via ingestion of unpasteurized dairy products or direct contact with infected animals.
- Brucella species are non-motile and non-spore-forming.
- They are catalase and oxidase positive.
- Urease activity is strong and rapid.
- Colonies are small, smooth, and non-hemolytic on blood agar.
- Diagnosis involves blood cultures, bone marrow cultures, or serological tests.
- Treatment typically includes a combination of antibiotics.
- Prevention strategies focus on pasteurization and vaccination of animals.
- Human-to-human transmission is extremely rare.
Scientific classification of Brucella
| Domain: | Bacteria |
| Phylum: | Pseudomonadota |
| Class: | Alphaproteobacteria |
| Order: | Hyphomicrobiales |
| Family: | Brucellaceae |
| Genus: | Brucella Meyer and Shaw 1920 (Approved Lists 1980) |
Characteristics of Brucella
- And Brucella are Gram‑negative coccobacilli, measuring approximately 0.5–0.7 µm × 0.6–1.5 µm
- non‑motile, non‑spore forming, and non‑encapsulated
- strictly aerobic, though some strains need 5–10 % CO₂ for optimal growth
- facultative intracellular pathogens, surviving and multiplying within macrophages
- catalase‑positive, oxidase‑positive (except rare species), and strong urease producers (urease rapid, often <2 h)
- partially acid‑fast, resist decolorization by weak acid (Ziehl–Neelsen)
- oxidize nitrates to nitrites and have nutritional demands—don’t grow on MacConkey agar, may need enriched media (blood or chocolate agar), slow‑growing (colonies often visible after 48 h)
- produce smooth or rough lipopolysaccharide (LPS) forms—smooth forms have O‑antigen chain, facilitating uptake into macrophages via lipid rafts
- genome composed of two circular chromosomes (Chr I and Chr II); Chr II is more accessory-rich and dynamic
- relatively small genome compared to environmental relatives, reflecting adaptation to intracellular lifestyle
- pathogens causing brucellosis in animals and humans (B. melitensis, B. abortus, B. suis, B. canis)
- So, Brucella are small, non‑motile coccobacilli; oxidase, catalase, urease positive; strictly aerobic; facultative intracellular; slow‑growing; require enriched media; possess LPS variants important in virulence; two‑chromosome genome; adapted to survive in macrophages.
Geographical Distribution of Brucellosis
- Brucellosis is common in some parts of the world, however the rates of infection vary.
- Brucellosis has been common in the Mediterranean region, which includes Greece and Italy.
- The Middle East, especially Iran, Iraq, and Egypt, has a lot of cases.
- Brucellosis is also a big problem in Central Asia, which includes Kazakhstan and Uzbekistan.
- Brucellosis is a big problem in Sub-Saharan Africa, especially East Africa.
- There are a lot of cases of human brucellosis in South Asia, which includes India and Pakistan.
- Southeast Asia, which includes China and Indonesia, has reported cases, while China has reported rare occurrences of B. suis.
- Brucellosis has been reported throughout Latin America, including Mexico, Peru, and Argentina. Argentina thinks that 10–13% of farm animals are afflicted.
- Vaccination and monitoring efforts have mostly kept brucellosis in animals under control in North America, which includes the US and Canada.
- Brucellosis is not a problem for livestock in Australia and New Zealand, and there is very little chance of it spreading to people.
- Brucellosis is mostly spread by touching contaminated animals or eating raw dairy products.
- To lower the danger of transmission, control techniques include vaccinating livestock, pasteurizing dairy products, and teaching the public about health.
Habitat of Brucella
- Brucella is a bacterium that must live inside cells.
- Its main home is inside host cells, especially macrophages and dendritic cells.
- The endoplasmic reticulum (ER) of host cells is where it replicates.
- Brucella lives in unique vacuoles that don’t mix with lysosomes.
- It can live in the environment if certain criteria are met.
- In chilly, damp places with organic matter, survival lasts longer.
- Brucella can stay alive in soil, manure, and water for a few months.
- It can stay alive in aborted fetal tissue for a long time.
- The bacteria don’t like heat, UV radiation, or acidic environments.
- Using bleach and alcohol to clean things is a good way to kill germs.
- Brucella can’t live on its own; it needs a host to reproduce.
Classification of Brucella
- Domain: Bacteria
- Phylum: Proteobacteria
- Class: Alphaproteobacteria
- Order: Rhizobiales
- Family: Brucellaceae
- Genus: Brucella
Species:
- B. melitensis – Sheep, goats, humans
- B. abortus – Cattle, buffalo, humans
- B. suis – Swine, humans
- B. canis – Dogs, foxes, coyotes, humans
- B. ovis – Rams
- B. neotomae – Desert wood rats
- B. ceti – Dolphins, porpoises
- B. pinnipedialis – Seals, sea lions
- B. microti – Field voles
- B. inopinata – Humans (rare)
- B. anthropi – Humans (formerly Ochrobactrum anthropi)
- B. vulpis – Foxes
The genus Brucella comprises both classical and novel species, with some formerly classified under the genus Ochrobactrum now included based on genetic analyses.
Morphology of Brucella
- Brucella are small, Gram-negative coccobacilli.
- Their size ranges from 0.5 to 0.7 µm in diameter and 0.6 to 1.5 µm in length.
- They appear singly, in pairs, or short chains.
- Brucella are non-motile and non-capsulated.
- They do not form spores.
- They are facultative intracellular pathogens.
- On Gram stain, they are faintly staining and may appear as fine sand grains.
- Colonies on blood agar are small, smooth, convex, and non-hemolytic.
- Growth requires special media like Castaneda medium with 5–10% CO₂.
- They are oxidase, catalase, and urease positive.
Human Infections Caused by Brucella Species
- Brucella infections cause brucellosis in both people and animals.
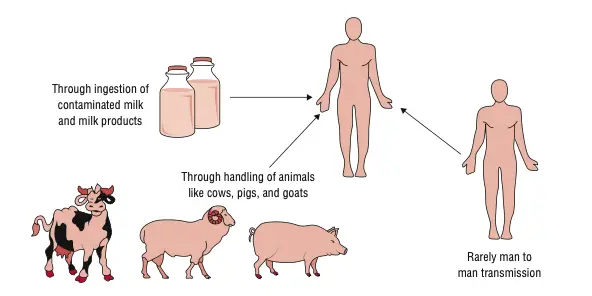
- Direct contact with diseased animals or eating animal products that are tainted can spread the disease.
- Infections can happen when you touch your skin, breathe in, or swallow anything.
- Cattle, sheep, goats, pigs, and dogs are all common reservoirs.
- The symptoms of brucellosis in people might be different and can last for a short time or a long time.
- Fever, sweating, feeling bad, not wanting to eat, headaches, and pain in the muscles and joints are all signs.
- Hepatosplenomegaly, lymphadenopathy, and arthritis are some of the things that can happen because of the disease.
- Endocarditis, osteomyelitis, neurobrucellosis, and chronic tiredness are some of the problems that can happen.
- Culture, serology, and molecular tests are used to make a diagnosis.
- Long-term antibiotic therapy, usually with doxycycline and rifampin or streptomycin, is needed for treatment.
- Vaccinating animals, pasteurizing dairy products, and giving workers safety gear are all ways to keep people safe.
- Brucellosis is a disease that must be reported in many countries because it is a public health concern.
Culture media and Culture Conditions for Brucella
Culture Media for Brucella
- Brucella Agar –
- Contains peptones, dextrose, and yeast extract.
- Supports growth of fastidious microorganisms.
- Farrell’s Medium –
- Selective medium prepared by adding antibiotics to serum dextrose agar.
- Inhibits growth of most contaminants, favoring Brucella species.
- Brucella Blood Agar (BRU) –
- Enriched medium containing casein, peptones, yeast extract, and dextrose.
- Supports growth of fastidious microorganisms.
- Trypticase Soy Agar with 5% Blood –
- General-purpose medium supporting growth of Brucella species.
- Chocolate Agar –
- Prepared by lysing red blood cells, releasing intracellular nutrients.
- Supports growth of fastidious organisms.
- MacConkey Agar –
- Differential medium for gram-negative bacteria.
- Brucella species typically show poor or no growth.
Culture Conditions for Brucella
- Temperature –
- Optimum: 37°C.
- Range: 20–40°C.
- pH –
- Optimum: 6.6–7.4.
- Range: 5.8–8.7.
- Atmosphere –
- Strictly aerobic.
- Some species require 5–10% CO₂ for optimal growth.
- Incubation Time –
- Colonies typically visible after 2–3 days.
- May require up to 10 days for full growth on selective media.
- Colony Morphology –
- Smooth strains: small, round, convex colonies.
- Rough or mucoid variants may form due to loss of O chains in lipopolysaccharide.
Biochemical Test of Brucella melitensis
- Gram Stain –
- Gram-negative coccobacilli.
- Motility –
- Non-motile.
- Spore Formation –
- Absent.
- Capsule –
- Absent.
- Oxidase –
- Positive.
- Catalase –
- Positive.
- Urease –
- Positive; hydrolysis detectable within 2 hours.
- H₂S Production –
- Negative.
- Dye Sensitivity –
- Growth on thionin 0.002%: Positive.
- Growth on thionin 0.004%: Negative.
- Growth on basic fuchsin 0.002%: Positive.
- Growth on basic fuchsin 0.004%: Negative.
- CO₂ Requirement –
- Non-requiring.
- Phage Sensitivity –
- Not destroyed by Tbilisi phage.
- Growth on Media –
- Growth on Brucella agar within 48 hours at 37°C.
- Colony Morphology –
- Smooth, glistening, and pin-point colonies.
- Gram Staining of Colonies –
- Small, gram-negative coccobacilli.
- Biochemical Misidentification –
- May be misidentified as Moraxella phenylpyruvica in API 20NE system.
Differential Features of Brucella Species
| Species | Natural Hosts | Urease | H₂S Production | Dye Sensitivity | Phage Sensitivity | CO₂ Requirement | Growth on MacConkey Agar | Colony Morphology |
|---|---|---|---|---|---|---|---|---|
| B. abortus | Cattle, buffaloes | Positive | Negative | Sensitive to thionin and basic fuchsin | Lysis by Tbilisi phage | Non-requiring | Poor or no growth | Smooth, glistening colonies |
| B. melitensis | Sheep, goats, camels | Positive | Negative | Sensitive to thionin and basic fuchsin | Lysis by Tbilisi phage | Non-requiring | Poor or no growth | Smooth, glistening colonies |
| B. suis | Pigs, wild boar, hares, reindeer | Positive | Negative | Sensitive to thionin and basic fuchsin | Lysis by Tbilisi phage | Non-requiring | Poor or no growth | Smooth, glistening colonies |
| B. canis | Dogs | Positive | Negative | Sensitive to thionin and basic fuchsin | Lysis by Tbilisi phage | Non-requiring | Poor or no growth | Smooth, glistening colonies |
| B. ovis | Sheep | Positive | Negative | Sensitive to thionin and basic fuchsin | Lysis by Tbilisi phage | Non-requiring | Poor or no growth | Smooth, glistening colonies |
| B. neotomae | Desert woodrats | Positive | Negative | Sensitive to thionin and basic fuchsin | Lysis by Tbilisi phage | Non-requiring | Poor or no growth | Smooth, glistening colonies |
| B. microti | Voles | Positive | Negative | Sensitive to thionin and basic fuchsin | Lysis by Tbilisi phage | Non-requiring | Poor or no growth | Smooth, glistening colonies |
| B. pinnipedialis | Seals, sea lions, walruses | Positive | Negative | Sensitive to thionin and basic fuchsin | Lysis by Tbilisi phage | Non-requiring | Poor or no growth | Smooth, glistening colonies |
| B. ceti | Dolphins, porpoises | Positive | Negative | Sensitive to thionin and basic fuchsin | Lysis by Tbilisi phage | Non-requiring | Poor or no growth | Smooth, glistening colonies |
| B. intermedia | Rarely in humans | Positive | Negative | Sensitive to thionin and basic fuchsin | Lysis by Tbilisi phage | Non-requiring | Poor or no growth | Smooth, glistening colonies |
| B. anthropi | Rarely in humans | Positive | Negative | Sensitive to thionin and basic fuchsin | Lysis by Tbilisi phage | Non-requiring | Poor or no growth | Smooth, glistening colonies |
Biotypes and Phage Types of Brucella Species
Biotypes of Brucella Species
- Brucella abortus
- Biovars 1–7, 9
- Found in cattle, buffaloes, and bison
- Biovar 3 is prevalent in Nigeria
- Brucella melitensis
- Biovars 1–3
- Common in sheep and goats
- Biovar 1 is most pathogenic to humans
- Brucella suis
- Biovars 1–5
- Biovar 2 infects wild boars and hares in Europe
- Biovar 5 is rare, found in rodents in the Northern Caucasus and South West Siberia
- Brucella canis
- Considered a stable rough mutant of B. suis biovar 1
- Causes brucellosis in dogs
Phage Types of Brucella
- Tbilisi (Tb)
- Isolated in 1955
- Used for typing B. abortus and B. melitensis
- Weybridge (Wb)
- Used for typing B. abortus and B. melitensis
- Firenze (Fi)
- Used for typing B. abortus and B. melitensis
- Berkeley 2 (Bk2)
- Used for typing B. abortus and B. melitensis
- Izatnagar 1 (Iz1)
- Used for typing rough Brucella strains
- Rough-specific phages (R/C)
- Target rough Brucella strains
- Rough phage (R)
- Used for typing rough Brucella strains
Note: Phage typing is a traditional method and may not always align with molecular typing results due to strain variability and phenotypic changes.
Cell Wall Components and Antigenic Structure of Brucella Species
- Cell Wall Type – Gram-negative cell wall structure
- Outer membrane, peptidoglycan layer, inner membrane
- More resistant to host enzymes due to smooth LPS
- Outer Membrane Components –
- Lipopolysaccharide (LPS) – major virulence factor
- Composed of lipid A, core oligosaccharide, and O-polysaccharide (O-side chain)
- Smooth strains (S) like B. abortus, B. melitensis, B. suis have complete O-antigen
- Rough strains (R) like B. ovis, B. canis lack or have defective O-antigen
- Prevents activation of innate immunity (TLR4 evasion)
- Outer Membrane Proteins (OMPs) –
- Omp1, Omp2a, Omp2b, Omp25, Omp31
- Help in adhesion, invasion, intracellular survival
- Omp25 mutation reduces virulence
- Porins – control passage of small molecules
- Phosphatidylcholine – rare in prokaryotes; helps mimic host cell membranes
- Lipopolysaccharide (LPS) – major virulence factor
- Peptidoglycan Layer –
- Thin compared to Gram-positives
- Contains diaminopimelic acid (DAP)
- Provides structural support and shape
- Cytoplasmic Membrane –
- Phospholipid bilayer
- Transport proteins, electron transport components
- Periplasmic Space –
- Contains enzymes like beta-lactamase
- Storage of virulence-related factors
- Antigenic Structures –
- O-polysaccharide (OPS) – immunodominant antigen in smooth strains
- Basis of S-R phase variation
- Target of Brucella-specific antibodies (e.g. in agglutination tests)
- A and M antigens – used to differentiate B. abortus (A > M) and B. melitensis (M > A)
- Serological classification based on relative proportions
- B. suis has intermediate A and M content
- Rough LPS antigens – detected in B. canis, B. ovis
- Weaker immunogenicity
- Detected by rough-specific agglutination tests
- Cytoplasmic antigens –
- Superoxide dismutase, heat shock proteins
- Intracellular targets in delayed-type hypersensitivity
- Capsular Polysaccharide – absent in Brucella
- No true capsule unlike other Gram-negative pathogens
- Lipid A – less endotoxic than E. coli
- Helps avoid triggering strong inflammatory responses
- O-polysaccharide (OPS) – immunodominant antigen in smooth strains
Virulence Factors of Brucella Species
And here are the key virulence factors of Brucella species:
- Lipopolysaccharide (LPS)
- smooth‑type LPS (LPS‑S) with full‑length O‑antigen enables stealth entry via lipid‑rafts and avoids phagolysosomal fusion
- O‑chain suppresses host apoptosis and evades immune detection
- Type IV secretion system (VirB T4SS)
- VirB complex injects effectors that reprogram intracellular trafficking
- essential for maturation of Brucella‑containing vacuole (BCV) and replication in the endoplasmic reticulum
- Two‑component regulatory system BvrR/BvrS
- controls outer‑membrane protein expression
- coordinates entry into host cells and intracellular survival
- Cyclic β‑1,2‑glucans
- modulate membrane osmotic conditions
- required for BCV formation and intracellular persistence
- Phosphatidylcholine synthesis
- eukaryotic‑like membrane lipid
- supports proper intracellular trafficking and virulence
- RNA chaperone Hfq and sRNAs
- Hfq regulates VirB operon and BabR regulator
- sRNAs modulate intracellular survival and stress response
- Quorum‑sensing regulator BlxR & flagellar proteins (e.g., FliK)
- BlxR controls expression of VirB and flagellar genes
- FliK implicated in immune modulation and full virulence
- Acid‑stress and urease (nikA gene) systems
- survive acidic phagosome via acid‑induced VirB and DnaK
- nikA‑mediated nickel uptake enables urease activity, buffering pH
- Absence of classical toxins or exotoxins
- Brucella lacks exotoxins, cytolysins, large plasmids, fimbriae—one of its stealth strategies
Pathogenesis of Brucellosis
- Step 1 – Entry into host
- Infection usually through ingestion (raw milk), inhalation (aerosols), or skin abrasions
- Brucella penetrates mucous membranes or damaged skin
- Step 2 – Phagocytosis by host cells
- Brucella is taken up by macrophages, dendritic cells, and epithelial cells
- Enters via lipid raft–mediated endocytosis to avoid typical phagolysosomal degradation
- Step 3 – Formation of Brucella-containing vacuole (BCV)
- Inside phagocytes, Brucella is enclosed in BCV
- Initially traffics through early endosomes and avoids fusion with lysosomes
- Step 4 – Intracellular survival and replication
- VirB type IV secretion system secretes effector proteins
- BCV interacts with endoplasmic reticulum (ER) and forms ER-derived replicative niche
- Brucella replicates in this modified compartment protected from host defenses
- Step 5 – Modulation of host immune response
- LPS (smooth type) suppresses TLR signaling, avoids strong immune activation
- Inhibits apoptosis of infected cells
- Reduces antigen presentation by MHC molecules
- Step 6 – Systemic dissemination
- Infected macrophages carry Brucella to regional lymph nodes
- From there, bacteria enter the bloodstream → bacteremia
- Localize in organs rich in reticuloendothelial cells (liver, spleen, bone marrow)
- Step 7 – Granuloma formation
- Chronic infection leads to granulomatous inflammation
- Brucella persists inside macrophages, sometimes for months or years
- Step 8 – Clinical manifestation
- Symptoms arise from inflammation, organ damage, and immune response
- Includes undulant fever, joint pain, hepatosplenomegaly, and fatigue
- Step 9 – Chronicity and relapse
- Due to intracellular persistence and immune evasion, relapses common
- Bacteria may reactivate from latent foci especially if untreated properly
Clinical Syndromes of Brucellosis
- fever (often undulant, intermittent, spiking in afternoon) – seen in ~80–90% of cases
- profuse sweating (sometimes with a foul/moldy odor)
- fatigue, malaise, anorexia, weight loss
- headache and myalgia
- arthralgia and arthritis – large joints, sacroiliitis, spondylitis; seen in 50–80%
- hepatomegaly and splenomegaly (with GI complaints like abdominal pain, nausea, dyspepsia)
- epididymo‑orchitis in men – up to ~10–20%, swelling and pain of testicles
- endocarditis – rare but most serious complication, main cause of mortality
- neurobrucellosis – includes meningitis, encephalitis, radiculopathy, cranial nerve palsies, psychiatric signs like depression, confusion
- genitourinary infection – urinary tract involvement, glomerulonephritis
- hematologic findings – anemia, leukopenia, thrombocytopenia occasionally
- cutaneous or pulmonary involvement – uncommon but possible (pneumonia, skin lesions)
And so, brucellosis presents as a multi-system disease with recurring fever and systemic signs, with focal complications in joints, heart, nervous, and genitourinary systems.
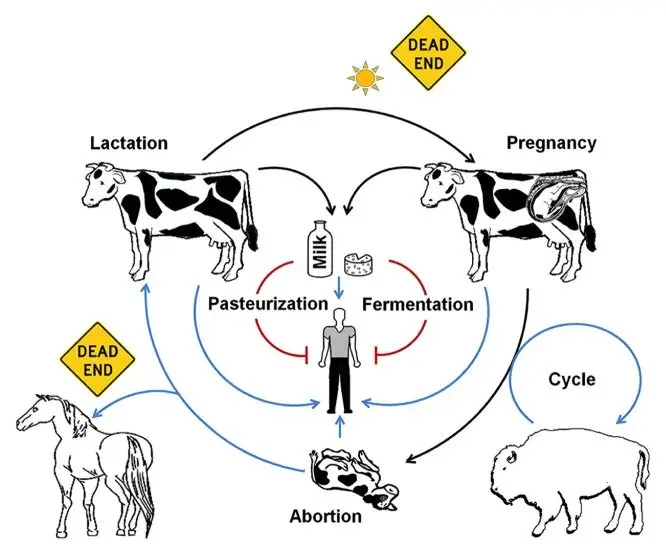
Reservoir, Source, and Transmission of Brucellosis
- Reservoir in nature
- livestock species: cattle (B. abortus), goats and sheep (B. melitensis), pigs (B. suis)
- dogs (B. canis), wild rodents (B. neotomae, B. microti), sheep (B. ovis)
- marine mammals (B. ceti, B. pinnipedialis), wildlife such as bison, elk, deer, wild boar
- Source of human infection
- unpasteurized dairy products (milk, cheese) from infected animals
- raw or undercooked meat from infected livestock or wildlife
- direct contact with infected animal tissues (placenta, fetus, genital secretions) during veterinary work, farming, slaughtering
- Transmission routes
- ingestion of contaminated food or drink
- inhalation of contaminated aerosols, especially in labs or abattoirs
- direct inoculation via skin abrasions or mucous membranes
- less common routes: conjunctival contact, blood transfusion, transplacental, sexual transmission (rare)
- Occupational exposure risk groups
- farmers, veterinarians, slaughterhouse and laboratory workers due to handling infected animals or specimens
- hunters and consumers of game meat infected by wildlife
- Wildlife reservoir dynamics
- wild bison, elk, deer, wild boar maintain B. abortus and spill back to livestock
- marine mammals transmit B. ceti via reproductive fluids and vertical routes; occasional human cases
So brucellosis is a zoonotic disease maintained in animal reservoirs, transmitted to humans via ingestion, inhalation, or direct contact with infected animal products or tissues.
Brucellosis Transmission Process to Humans
- humans acquire brucellosis through a defined zoonotic process
- Step 1 – reservoir in animals
- Brucella spp. persist in livestock (cattle, goats, sheep, pigs, dogs) and wildlife (bison, elk, deer) (B. abortus, B. melitensis, B. suis, B. canis)
- Step 2 – bacterial shedding by infected animals
- Brucella is released in milk, uterine/placental tissues, fetal fluids, urine, and genital secretions, especially during birthing or abortions
- Step 3 – contamination of products/environment
- Raw milk and fresh dairy products become contaminated
- Environment in farms/slaughterhouses/wildlife areas is laden with bacteria from secretions and fluids
- Step 4 – human exposure via ingestion
- Most common route: consuming unpasteurized milk, cheese, raw/undercooked meat including game
- Step 5 – human exposure via inhalation
- Breathing in contaminated aerosols (dust, animal birthing fluids, lab cultures)
- Highly infectious via small doses (10–100 organisms)
- Step 6 – direct inoculation through contact
- Bacteria enter through skin cuts/abrasions or mucous membranes (eyes, nose, mouth) during handling of infected animals/products
- Step 7 – occasional human-to-human transmission
- Rare, but documented: transplacental (mother → fetus), breastfeeding, sexual contact, blood transfusion, organ transplantation
- Step 8 – initial infection and intracellular survival
- Once inside body, Brucella is engulfed by phagocytes
- Avoids degradation and replicates intracellularly, spreading through lymphatic and reticuloendothelial systems
- Step 9 – bacteremia and systemic spread
- Bacteria enter bloodstream, reaching spleen, liver, bone marrow, lymph nodes, and reproductive organs
- Step 10 – clinical disease manifestation
- Causes undulant fever, night sweats, fatigue, arthralgia, hepatosplenomegaly, and potential focal complications (arthritis, endocarditis, neurobrucellosis)
So, transmission follows this clear pathway: animal reservoir → bacterial shedding → contamination → human acquisition via ingestion/inhalation/contact → intracellular survival → systemic spread and disease.
Laboratory Diagnosis of Brucellosis
Brucellosis is notoriously difficult to diagnose clinically due to its wide variety of symptoms. Therefore, laboratory diagnosis plays a crucial role in confirming the presence of the infection. Several methods are used to detect Brucella organisms or antibodies in the patient, each with varying sensitivity and specificity.
- Specimen Collection:
- Blood is the preferred specimen for both culture and serological tests.
- Bone marrow and, in some cases, synovial fluid or pleural fluid are collected for culture, as they can provide more reliable results than blood cultures.
- Other potential specimens include liver, lymph nodes, cerebrospinal fluid (CSF), urine, sputum, breast milk, vaginal discharge, and seminal fluid, though these are less commonly used for isolation.
- Microscopy:
- Gram staining is not effective for detecting Brucella due to the bacterium’s small size and its intracellular location.
- Culture Methods:
- The culture of Brucella from blood or other clinical specimens remains the most definitive method for diagnosing brucellosis.
- Blood cultures involve collecting 5–10 mL of blood in a broth (such as serum dextrose broth or trypticase soy broth) and incubating at 37°C under 5–10% CO2.
- Cultures should be subcultured onto solid media after 4 days and checked every 3–5 days for up to 8 weeks before being declared negative.
- Bone marrow cultures are more sensitive than blood cultures, often yielding positive results when blood cultures are negative. Synovial fluid cultures are positive in about 50% of patients.
- Identification of Brucella Colonies:
- Microscopic examination of Gram-stained smears, along with colony morphology and biochemical tests, helps identify Brucella colonies.
- Specific antibrucella sera are used to confirm the identity of the bacteria.
- Serodiagnosis:
- Serological tests are key for diagnosing subclinical, acute, and chronic brucellosis by detecting specific antibodies in the patient’s serum.
- IgM antibodies appear 7–10 days after infection and persist for up to 3 months, while IgG and IgA antibodies appear later and can persist for years.
- A significant rise in antibody titers (usually fourfold) or a single high titer of 1:160 is indicative of brucellosis.
- B. abortus antigen is used in tests like agglutination because it can detect antibodies against multiple Brucella species, including B. melitensis and B. suis, but not B. canis, which requires a specific antigen.
- Common Serological Tests:
- Standard Tube Agglutination Test (STA): This is the most widely used test for diagnosing brucellosis. It detects antibodies against the lipopolysaccharide (LPS) component of Brucella.
- Positive results are indicated by a titer of 1:160 or a fourfold rise in convalescent sera. This test is useful for detecting brucellosis caused by B. abortus, B. melitensis, and B. suis but not B. canis.
- Modified Tube Agglutination Test (MTAT): This test adds 2-mercaptoethanol to break down IgM antibodies, allowing detection of IgG antibodies. It is helpful for diagnosing brucellosis during the convalescent phase or in cases of relapse or persistent infection.
- Indirect Immunofluorescent Assay: A sensitive test that detects brucella antibodies, even in cases that are negative for the agglutination test.
- Enzyme-Linked Immunosorbent Assay (ELISA): This is the most sensitive test, especially for detecting IgM, IgA, and IgG antibodies during acute and chronic stages. It is also useful for monitoring neurobrucellosis by detecting antibodies in CSF.
- Standard Tube Agglutination Test (STA): This is the most widely used test for diagnosing brucellosis. It detects antibodies against the lipopolysaccharide (LPS) component of Brucella.
- Challenges with Serological Testing:
- Blocking Antibodies: These can cause false positives. They can be addressed by heating the serum or using a diluent such as saline or Coombs’ test.
- Prozone Phenomenon: This occurs when high levels of antibodies inhibit agglutination, causing a false negative result. Diluting the serum helps avoid this issue.
- Cross-reactivity: Antibodies against other bacteria like Vibrio cholerae, Yersinia enterocolitica, Francisella tularensis, and Salmonella can cause false positives due to similar LPS structures. These can be reduced by absorbing cholera-induced antibodies.
- Brucella Skin Test:
- This is a delayed type hypersensitivity reaction where brucellin (a protein extract) is injected intradermally.
- A positive result shows erythema and induration of at least 6 mm within 24 hours, but this test is only positive in chronic brucellosis and negative in the acute phase.
- Other Diagnostic Features:
- Bone marrow examination often reveals erythrophagocytosis.
- CSF cultures from patients with neurobrucellosis show signs like pleocytosis, increased protein, and hypoglycorrhea.
- Anemia, thrombocytopenia, and pancytopenia are common hematological findings in brucellosis patients.
- Diagnosis in Animals:
- In animals, the diagnosis mirrors that used for humans, with the added option of testing milk and urine from infected animals.
- Rapid tests like the latex agglutination and Rose Bengal card tests are commonly used to diagnose brucellosis in cattle.
- The Milk Ring Test is a screening method where B. abortus or B. melitensis antigens are mixed with milk to detect antibodies. A positive test forms a blue ring at the top of the milk, indicating the presence of Brucella infection.
Treatment of Brucellosis
- First-line therapy
- doxycycline 100 mg orally twice daily for 45 days
- streptomycin 1 g intramuscularly daily for 14 days
- or gentamicin 5 mg/kg intramuscularly once daily for 7 days as an alternative to streptomycin
- combination therapy reduces relapse risk and enhances efficacy
- rifampin can be added for certain cases, especially neurobrucellosis
- monotherapy with doxycycline alone is less effective and not recommended for acute or chronic cases
- triple therapy (doxycycline + rifampin + co-trimoxazole) is used for neurobrucellosis
- Chronic or complicated brucellosis
- combination of doxycycline and rifampin for 45 days
- or doxycycline with gentamicin for the first 5 days, followed by doxycycline and rifampin for the remaining duration
- for endocarditis, a regimen of doxycycline, rifampin, and trimethoprim-sulfamethoxazole for at least 4 weeks, followed by at least 2–3 active agents (excluding aminoglycosides) for another 8–12 weeks, is preferred
- surgical intervention may be necessary for abscesses or resistant cases
- Pregnant individuals
- rifampin 15–20 mg/kg per day (maximum 600–900 mg/day) for 6 weeks is the recommended antimicrobial therapy
- rifampin is classified as a FDA Pregnancy Category C drug, indicating potential fetal risks based on animal studies, but it is commonly used in pregnant individuals when necessary
- Pediatric considerations
- for children under 8 years old, doxycycline is generally contraindicated
- preferred regimen includes rifampin with cotrimoxazole for 45 days
- an alternative regimen consists of rifampin for 45 days with gentamicin 5–6 mg/kg/day for the first 5 days
- Supportive care
- antipyretics and analgesics for symptom management
- corticosteroids may be considered for symptomatic Brucella meningitis, although scientific evidence supporting their use is lacking
- no consensus exists on optimal dosing, frequency, or duration of corticosteroid therapy
- Relapse and recurrence
- relapse rates can be reduced with appropriate combination therapy
- recurrence may occur in 5–10% of patients, particularly in cases of neurobrucellosis or endocarditis
- prolonged or repeated courses of antibiotics may be necessary for persistent or recurrent infections
- Treatment duration
- standard treatment duration is 6 weeks for uncomplicated cases
- longer durations may be required for complicated cases, such as neurobrucellosis or endocarditis
- Monitoring and follow-up
- regular monitoring of liver function tests and complete blood counts during treatment
- follow-up cultures may be necessary to confirm eradication of the infection
- serological testing can be used to assess response to treatment and detect potential relapses
- Prevention of relapse
- adherence to the full course of prescribed antibiotics is crucial to prevent relapse
- patients should be educated on the importance of completing the entire treatment regimen, even if symptoms improve before completion
- Special considerations
- in cases of neurobrucellosis, a triple therapy regimen (doxycycline + rifampin + co-trimoxazole) is often employed
- for endocarditis, a combination of doxycycline, rifampin, and trimethoprim-sulfamethoxazole is recommended, with potential surgical intervention if necessary
- Alternative therapies
- while some studies have explored the use of other antibiotics, such as fluoroquinolones, for brucellosis treatment, they are not routinely recommended due to concerns about efficacy and resistance patterns
- Adjunctive therapies
- no specific adjunctive therapies have been proven to enhance the effectiveness of antibiotic treatment for brucellosis
- supportive care remains the cornerstone of management for symptomatic relief
- Vaccination
- currently, no effective vaccine is available for humans
- vaccination programs in livestock (e.g., Brucella abortus strain 19 for cattle) are essential for controlling the spread of brucellosis and reducing human cases
- Public health measures
- education on the risks of consuming unpasteurized dairy products and undercooked meat
- implementation of control measures in livestock populations, including vaccination and culling of infected animals
- occupational safety protocols for individuals at risk, such as farmers and laboratory personnel, to prevent exposure to Brucella spp.
Prevention and Control of Brucellosis
- Animal Vaccination
- B. abortus strain 19 or RB51 for cattle
- B. melitensis Rev-1 for goats and sheep
- B. suis strain 2 for pigs
- B. canis strain RB51 for dogs
- B. ovis for sheep (no human vaccine available)
- WHO recommendation: vaccination in enzootic areas with high prevalence rates
- Surveillance and Herd Management
- Regular serological testing of livestock
- Culling of infected animals to prevent spread
- Implement quarantine and movement controls for affected herds
- Pasteurization of Dairy Products
- Heat milk to 70°C (160°F) for 2–3 seconds
- Ensure all dairy products are pasteurized before consumption
- WHO recommendation: pasteurization is the most effective method to eliminate Brucella bacteria in milk
- Food Safety Practices
- Avoid consumption of raw or undercooked meat, especially from game animals
- Proper cooking of meat to safe internal temperatures
- Safe handling of animal tissues during butchering and processing
- Occupational Safety Measures
- Use personal protective equipment (PPE) such as gloves, masks, and goggles when handling animals or animal products
- Implement biosafety protocols in laboratories working with Brucella spp.
- Post-exposure prophylaxis with doxycycline and rifampin for individuals with significant exposure risks
- Public Awareness and Education
- Educate communities about the risks of brucellosis and preventive measures
- Promote hand hygiene, safe food handling, and vaccination practices
- Engage in community outreach to raise awareness and encourage participation in control programs
- One Health Approach
- Integrate human, animal, and environmental health efforts to control brucellosis
- Collaborate among public health, veterinary, and environmental sectors
- Implement cross-sectoral coordination for effective disease management
These measures, when implemented collectively, can significantly reduce the incidence and spread of brucellosis in both humans and animals.
- Alton GG, Forsyth JRL. Brucella. In: Baron S, editor. Medical Microbiology. 4th edition. Galveston (TX): University of Texas Medical Branch at Galveston; 1996. Chapter 28. Available from: https://www.ncbi.nlm.nih.gov/books/NBK8572/
- Carvalho TPD,Silva LAD, Castanheira TLL,Souza TDD, Paixão TAD, Lazaro-Anton L, Tsolis RM, Santos RL,2023.Cell and Tissue Tropism of Brucella spp.. Infect Immun91:e00062-23.https://doi.org/10.1128/iai.00062-23
- https://emedicine.medscape.com/article/213430-overview
- https://www.woah.org/en/disease/brucellosis/
- https://www.webmd.com/a-to-z-guides/brucellosis-symptoms-treatment
- https://www.sciencedirect.com/topics/immunology-and-microbiology/brucella
- https://medlineplus.gov/ency/article/000597.htm
- https://www.msdmanuals.com/professional/infectious-diseases/gram-negative-bacilli/brucellosis
- https://www.cdc.gov/brucellosis/pdf/brucellosi-reference-guide.pdf
- https://www.mayoclinic.org/diseases-conditions/brucellosis/symptoms-causes/syc-20351738
- https://my.clevelandclinic.org/health/diseases/17886-brucellosis
- https://www.cdc.gov/brucellosis/about/index.html
- https://www.who.int/news-room/fact-sheets/detail/brucellosis
- https://emedicine.medscape.com/article/213430-overview
- https://en.wikipedia.org/wiki/Brucella
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.