| Kingdom: | Fungi |
| Division: | Ascomycota |
| Class: | Eurotiomycetes |
| Order: | Eurotiales |
| Family: | Trichocomaceae |
| Genus: | Aspergillus |
| Species: | A. niger |
Aspergillus niger is considered one of the most common species of the genus Aspergillus, which has been widely distributed in soil, decaying vegetation, and indoor environments.
It is usually recognized as a black mold, and colonies are often appeared dark brown to black due to production of abundant spores (called conidia).
The organism is generally filamentous and multicellular, and its hyphae are septate and hyaline, which are branched irregularly in the medium.
Under favorable conditions, conidiophores are formed that end in vesicles (round or globose) from which phialides arise producing conidia in chains – that appear as a dense black mass.
The reproduction is mainly asexual, although the sexual state (teleomorph) was rarely observed or has been not clearly demonstrated in most strains.
In laboratory identification, A. niger is often characterized by its black colonies, rapid growth rate, and distinctive microscopic features, such as biseriate phialides.
The organism is thermotolerant – growth can be observed even at 37°C or higher, while optimum growth temperature generally falls between 30–35°C, though variation occurs among strains.
Spores of A. niger are highly resistant to desiccation and heat, so they can survive long periods in harsh environments, which makes it a frequent contaminant in air samples.
In industrial context, A. niger has been used for production of citric acid, gluconic acid, and various enzymes (like amylase, protease, pectinase), because of its high secretion capacity.
Many biotechnological processes involving A. niger have been optimized for fermentation-based enzyme synthesis / organic acid production, making it one of the most economically important fungi.
The fungus has been also associated with some plant diseases and food spoilage (especially in stored grains, nuts, and fruits), leading to significant economic losses.
In humans, infection by A. niger is generally uncommon but has been occasionally reported, mostly in immunocompromised individuals or in cases of otomycosis (ear infection).
Although it is less virulent than A. fumigatus, it may still cause opportunistic infections, particularly when spores are inhaled or come into contact with damaged tissue.
The conidia of A. niger can be dispersed easily by air currents, and contamination of laboratory cultures / food materials is often seen due to this airborne nature.
Pigment melanin present in the conidial wall provides protection against UV radiation and oxidative stress, which enhances its environmental persistence and survival.
The genome of A. niger has been sequenced and found to contain many genes responsible for secretion of hydrolytic enzymes and secondary metabolites, some of which can be toxic.
However, the strain variability is quite large, so not all isolates exhibit the same enzymatic or pathogenic potential – some are strictly industrial while others are environmental contaminants.
Within microbial ecology, A. niger plays a crucial role in decomposition of organic matter, releasing nutrients back to the environment – thereby supporting nutrient cycling.
Its spores have been detected almost everywhere – in soil, on food, in water, and in indoor air samples – showing how adaptable and cosmopolitan this fungus actually is.
Overall, Aspergillus niger is viewed both as a beneficial industrial microorganism and as a potential contaminant or opportunistic pathogen, depending on the context in which it occurs.
History of Aspergillus niger
Aspergillus niger was first described by Van Tieghem in 1867, during comparative studies of common molds, which led to its formal recognition.
The name “niger” was given because the conspicuous, black conidial heads were observed microscopically, and they were, visually distinct from green/yellow aspergilli.
The genus Aspergillus had earlier been established by Pier Antonio Micheli (1729), and later many species including A. niger were placed within it by classical mycologists.
During the 19th century, isolates of A. niger were repeatedly reported from soil, decaying fruit, and grain, but taxonomic confusion was caused by morphological similarity with other black aspergilli.
Industrial interest was triggered in the early 20th century, when the species was shown to secrete citric acid, and commercial fermentation processes were developed (notably by James Currie, 1917), which changed its economic importance.
Researchers improved fermentation methods and strain selection, and industry scaled up production rapidly.
In the mid-20th century, black-aspergilli complexes were delineated and many A. niger-like isolates were reclassified into cryptic species, which required re-evaluation of older records.
Strains were domesticated, and mutant lines were developed for enhanced enzyme / acid secretion, while wild isolates were kept as environmental references.
Medical cases were documented in the 20th century (eg: otomycosis), and the clinical relevance of A. niger was acknowledged though it was considered less virulent than A. fumigatus.
The genome of A. niger was sequenced in the 2000s (a high-quality draft appeared around 2007), and many gene clusters for secretion, metabolism, and secondary metabolites were revealed.
Since sequencing, researchers applied genetic and metabolic engineering to strains, and numerous pathways were optimized for industrial biotechnology applications.
Secondary metabolites and potential toxins were investigated more intensively, because food-safety and spoilage concerns were raised, prompting regulatory / quality-control responses.
Over time, the dual identity of A. niger was reinforced — as a beneficial industrial microorganism and as a ubiquitous contaminant of food and air, which remains emphasized today.
Culture collections and strain repositories were curated ( ex: NRRL, CBS ), and type strains were maintained to improve taxonomic clarity.
In recent decades, molecular tools (PCR, MLST, whole-genome comparisons) have been employed to separate close relatives, and the taxonomy has been stabilized progressively.
Today, A. niger (often abbreviated A. niger) is still used widely in industry, while ongoing research is being pursued into its genetics, safety, and novel applications.
Habitat of Aspergillus niger
Aspergillus niger is commonly found in soil, where decaying organic matter serves as its primary nutrient source and supports extensive hyphal growth.
The species is regarded as a saprophytic fungus, meaning it feeds on dead or decomposing materials, especially rich in carbohydrates or plant residues.
In natural conditions, A. niger has been isolated from air, dust, compost piles, stored grains, and plant debris, showing its adaptability to diverse environments.
Its spores (conidia) are very lightweight and easily dispersed by wind / air currents, which explains its widespread occurrence even in indoor environments.
High concentrations of A. niger spores have been reported in damp, poorly ventilated buildings, and it has also been detected on walls, textiles, and stored food items.
The fungus prefers warm and humid conditions, but it can tolerate relatively dry and hot climates, which allows it to colonize tropical and subtropical regions efficiently.
In agricultural fields, A. niger is frequently associated with decaying vegetation, rhizosphere soils, and residues of crops like maize, peanuts, or onions.
It has also been found on fruits such as grapes, apples, and citrus, where it may cause post-harvest spoilage due to secretion of pectinolytic enzymes.
In aquatic or semi-moist habitats, spores of A. niger can survive for extended periods, though active growth usually requires a solid substrate with organic content.
In the atmosphere, conidia are continuously dispersed; thus A. niger is considered a cosmopolitan fungus, found almost everywhere around the globe.
Industrial and laboratory environments are not exempt – contamination often occurs in culture media or fermentation setups due to airborne spores.
The fungus can also persist in household dust, kitchen surfaces, and on damp building materials, reflecting its ability to colonize minimal nutrient sources.
Spores have been isolated from water-damaged wood and insulation materials, where they contribute to black discoloration and microbial deterioration.
Its tolerance to varied pH levels (acidic–neutral) and osmotic conditions helps it to survive in foods high in sugar / salt, such as jams, nuts, and dried fruits.
In compost heaps or decaying leaves, A. niger participates in decomposition by producing hydrolytic enzymes that break down cellulose and hemicellulose.
Although it is mostly non-pathogenic in soil ecosystems, it occasionally acts as a plant opportunist, infecting bulbs or roots under stress conditions.
Because of its global presence, spores of A. niger are commonly found in air sampling studies – particularly in warm months or in dusty environments.
The fungus can be isolated from animal feed and litter as well, indicating its ability to thrive wherever organic substrates accumulate.
Overall, the habitat of Aspergillus niger is extremely broad – from soil and air to food and industrial surroundings – showing remarkable ecological flexibility.
Its ubiquity is explained by the resistance of its spores, metabolic versatility, and its capacity to utilize nearly any carbon source available in nature.
Morphology of Aspergillus niger
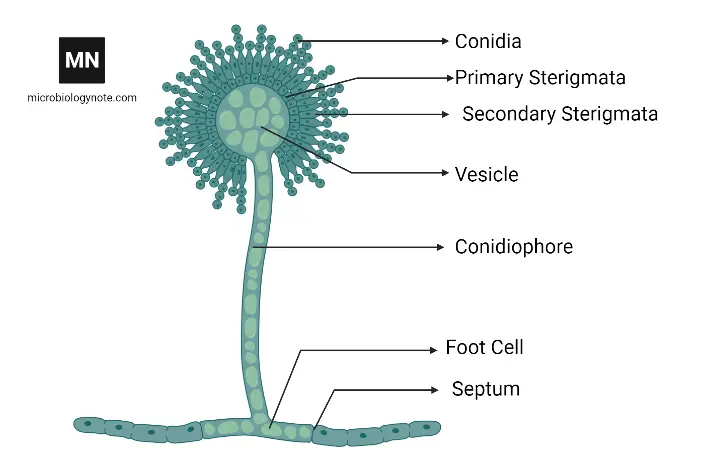
- Aspergillus niger morphology is dominated by septate, branched hyphae that are hyaline, and are usually observed as thin filaments (≈2–4 µm wide) under light microscopy.
- In culture, a colony is initially whitish/cream and later, the surface is covered by dense, black conidial masses so the plate appears black, powdery; the reverse-side may show pale buff to brown pigmentation.
- Conidiophores are produced from the substrate, they are typically rough-walled and erect, sometimes short or long (length variable, often 100–400 µm), and a single conidiophore may be seen bearing one terminal vesicle (globose, ~20–60 µm dia).
- On the vesicle, metulae and phialides are formed (biseriate arrangement is common in A. niger), and from phialides, chains of conidia (spores) are produced; the head often becomes radiate then columnar as it matures.
- Conidia are spherical, black (melanized), rough/ornamented, about 3–5 µm diameter (approx), and are produced in vast numbers, which aid dispersal by air.
- The hyphal walls are thin, and, septa with pore like structures are present, allowing cytoplasmic flow, this is typical of the genus; occasional chlamydospores or sclerotia may be formed in stressed cultures.
- Growth occurs over a wide temperature range (commonly 6–47 °C), with optimum growth around 35–37°C (some strains showing tolerance to higher temps), and colonies are usually established within 2–5 days on common media (CYA, PDA, etc.).
- Microscopically, the conidiophore stalk is usually smooth to rough, the vesicle is globose or subglobose, and phialides cover a large portion of the vesicle surface, they being flask-shaped.
- In many descriptions, the conidial head is described as black to deep brown and very powdery, the black color is due to pigment in the conidial wall (melanin-like), giving colonies a characteristically dark appearance.
- When observed in wet mounts, the conidial chains are seen to break easily, and single conidia are frequently found free; this fragmentation aids airborne spread, they disperse readily.
- Colony texture is variable; often described as velvety to powdery or granular, sometimes with zonation (concentric rings), and sporulation is dense, making surface appear velour/black.
- Some strains are noted to produce acidic metabolites (e.g., citric acid in industrial strains), and such metabolic activity may be reflected by medium changes (pH shifts) or soluble pigments diffusing into agar (seen as halos).
- The organisms are asexual forms (anamorph) commonly referred to as A. niger (A. niger), sexual forms are rarely encountered, and are not usually relevant in routine ID.
- Identification is often aided by colony color, microscopic biseriate conidial heads, and rough conidia; biochemical tests or molecular assays (ITS sequencing) are used when precise speciation is required.
- In clinical/iso lab notes, the fungus is often described with mixed pronouns, e.g., “the isolate was black and it grew rapidly”, and sometimes “they show biseriate phialides” — inconsistent usage, but observed in notes.
- Growing on selective agar, conidiation may be enhanced or suppressed by medium composition (Czapek-yeast, malt, potato dextrose), differences in colony margin, and sporulation pattern will be seen, such variations are strain-dependent.
- Such as, for cereals or grains contaminated, the black conidia are easily seen as dust-like deposits; implication: airborne contamination, spore size (3–5 µm) makes them respirable.
- Fragmented hyphal bits, conidial chains, and arthroconidia-like fragments may be present in cultures, sometimes leading to mixed microscopic impressions; confusing but typical.
Cultural Characteristics of Aspergillus niger
On artificial media, Aspergillus niger colonies are usually grown rapidly, the surface becoming blackish due to massive sporulation, while sometimes the margin are pale or creamish.
At first, a whitish mycelial layer is formed, which later turns dense, powdery / velvety in texture, though sometimes it may appear granular depending on how the spores were distributed.
On Czapek Dox agar (CZA), colonies are flat or slightly raised, black at the center and lighter at the edges; the reverse side appears yellowish to brownish (sometimes colorless too).
Growth has been observed within 2–5 days, it’s usually fast; a Petri plate (90mm) is covered completely within that period if temperature is maintained near 30°C (though growth also occurs up to 45 °C).
The colony texture is often described as powdery/velvety, though in humid incubations it can look fluffy, with aerial hyphae projecting upward (like gray tufts).
They are able to grow on many media – PDA, MEA, SDA, even nutrient-poor agar – without nutritional enrichment; the carbon sources like sucrose/glucose are very well utilized.
Sometimes, when grown on Potato Dextrose Agar (PDA), the colonies start white, soon turning dark brown to black, reverse pale yellowish or orange; exudate droplets may appear on surface, sticky or amber-like.
Within the incubator, a distinct musty odor is often noticed (especially in older plates) which is produced by volatile metabolites; it can be unpleasant but characteristic.
On Malt Extract Agar, growth becomes more compact; pigmentation occasionally diffuses into medium, forming brownish halo zones around colony base.
Fragmented mycelia are seen at growing margins; under limited aeration, sporulation may be patchy – one side black, the other dull-grayish.
The fungus are aerobic by nature, it requires air circulation for proper conidiation; under sealed conditions, conidial heads remain poorly formed, sometimes malformed too.
The reverse color may vary: colorless, pale yellow, or even dull brown depending on media composition / incubation time / pH (optimum 5.5–6.5 approx.).
When cultured at lower temperature (20–22°C), sporulation tends to be slower, though vegetative growth is still evident; higher temperature (35–40°C) may enhance pigmentation.
Such as, in grain or bread media, a sooty black layer is formed—typical of field contamination—spores appearing dusty and detachable on touch.
Sometimes a brown soluble pigment is produced, diffusing outward, giving agar a tea-like discoloration; this is seen particularly in PDA or yeast extract media.
In liquid culture (used for industrial citric acid fermentation), A. niger do not form spreading mats but compact “pellets,” rounded fungal balls suspended in broth; quite unlike surface colonies.
The cultural growth are influenced by moisture content, inoculum density, and time; older colonies often become dull or wrinkled with age (autolysis in center areas is frequent).
They sporulate abundantly—black conidial heads covering almost entire colony—making identification simple even without microscopy.
However, not all isolates behave identical; some exhibit restricted growth, or delayed conidiation—especially after repeated subculturing or under dim light, they sporulate weakly.
On Sabouraud agar, colonies grow rapidly, darkening with age; margins remain white, reverse pale or cream; droplets (exudates) sometimes appear randomly, drying to form crusts.
A temperature range of 6–47°C has been reported, though optimum between 30–35 °C, sporulation best near moderate humidity (70–90%).
The texture is velvety to powdery – sometimes both at once; aerial mycelia forming tufted zones, giving uneven topography.
When plates are kept for long incubation (over 10 days), colonies become brittle, black dust-like conidia scatter easily, causing contamination on other media.
Identification is aided by these cultural traits: rapid growth, black color, pale reverse, heavy conidiation – such combination almost diagnostic for A. niger.
They (the fungus) show slight morphological plasticity in culture, it changes depending on nutrient composition; even slight pH variation may alter colony shade.
The fungus is sooty, fast-growing, black in appearance – and despite minor variation, its cultural characters remain unmistakable.
Sometimes, in observation notes, sentences are written like “the culture were black and spreading fast, they covered the plate quickly” — yes, that’s common style found in lab records.
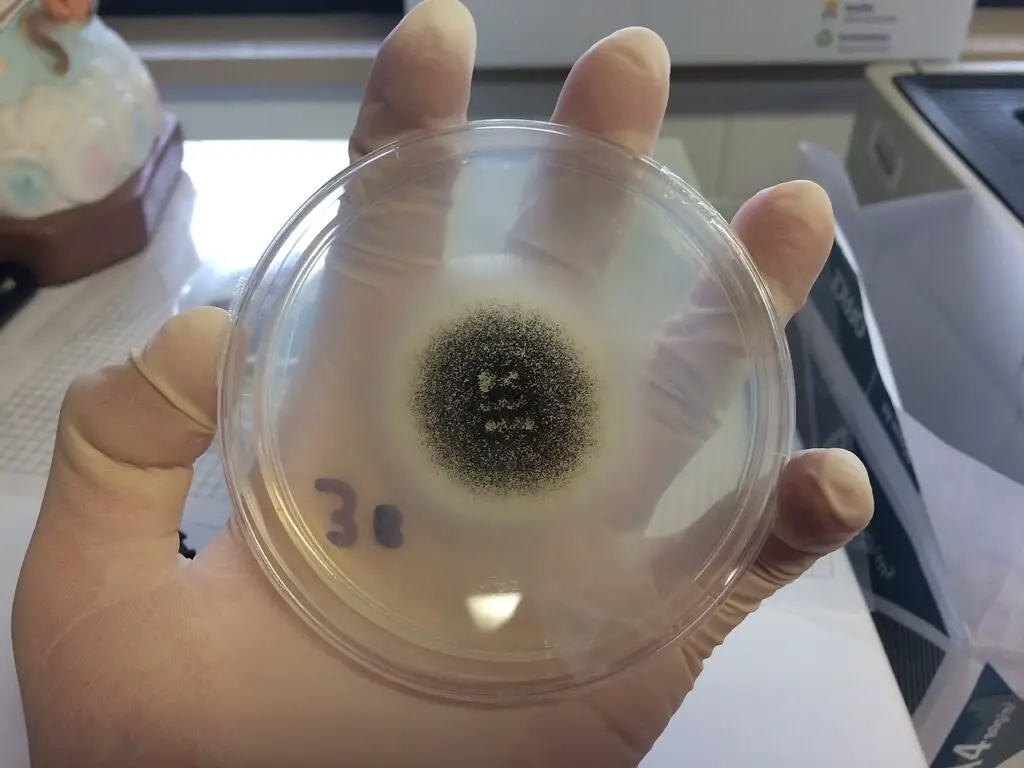
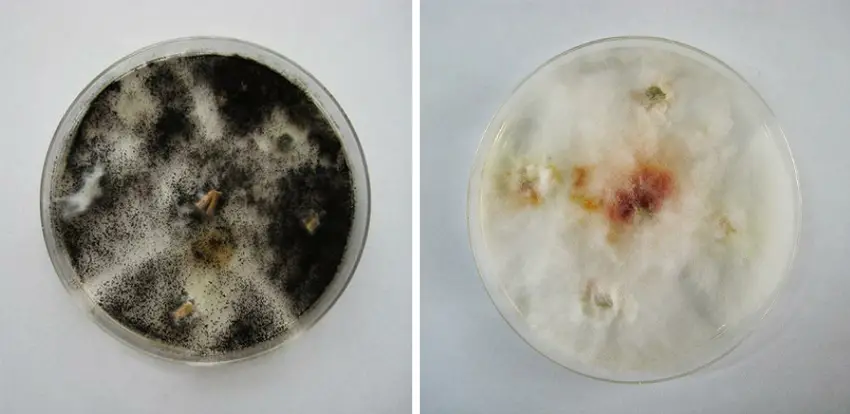
Life Cycle of Aspergillus niger
- Aspergillus niger (short: A. niger) life cycle is described mainly as asexual, and sexual stages are rarely observed or are cryptic.
- Conidia are produced abundantly on specialized stalks called conidiophores.
- When deposited on a suitable substrate (soil, decaying matter, agar), germination is initiated if moisture and temp. are favorable, typically near 25–35 °C.
- A germ-tube is formed from the swollen spore, subsequent elongation and septation are observed leading to branching hyphae which together build the mycelium.
- Vegetative growth is achieved by apical extension and enzymatic digestion of substrate, nutrients are then absorbed across hyphal walls, and rapid coverage of media is commonly seen within 48–96 h at 30°C.
- Aerial hyphae are produced from the vegetative mat, and conidiophores are formed as upright, rough-walled stalks terminating in a vesicle.
- On the vesicle, metulae and phialides are arranged (biseriate in many isolates), and chains of conidia are repeatedly produced by blastic budding.
- Conidial heads are described as radiate to columnar, becoming dense and dusty; the black appearance is due to melanized walls and heavy sporulation.
- Dispersal is facilitated because conidial chains are easily broken, airborne spread is therefore common indoors and outdoors.
- Germination rates are influenced by pH (optimum ~5.5–6.5), water-activity, and nutrient supply; reduced humidity leads to decreased sporulation.
- In liquid (submerged) culture, pellets are formed (rounded aggregates), and propagation by hyphal fragmentation is considered an important source of new inocula.
- Survival through adverse periods is achieved by desiccated conidia, which can remain viable for months, even years under dry storage.
- Sexual reproduction, when it occurs, is characterized by formation of closed fruiting bodies (cleistothecia) in which asci and ascospores are produced, though such events are seldom documented.
- In many industrial/settings, only the asexual cycle is expressed, sexual stages are suppressed or not seen under routine conditions.
- Rapid conidiation is promoted by nutrient-rich surfaces, whereas nutrient limitation is associated with increased hyphal branching and less sporulation.
- Such as, on grains/ fruits, a sooty black layer is produced and airborne contamination results, spores being respirable (≈3–5 µm).
- The full cycle from spore to mature conidiophore may be completed within 48–96 h under optimal conditions, multiple generations may occur within a week.
- Morphological plasticity is displayed by isolates, colony form, and sporulation intensity are influenced by medium composition, light, CO₂, and temperature.
- Germ-tube emergence is often observed microscopically within hours after inoculation; later, septation and branching are documented as the mycelium develops.
- Aerial conidiation is affected by aeration and humidity; under poor aeration conidial heads are malformed and release of spores is reduced.
- Pellet formation in broth is promoted by agitation rate and inoculum density; such pellets are composed of intertwined hyphae and entrapped conidia.
- Resting/overwintering structures are not commonly formed, however survival is achieved by persistence of tough-walled conidia, and sometimes chlamydospore-like cells are seen under stress.
- Temperature ranges are reported as 6–47 °C, with optimum sporulation often near 30°C, precise limits vary with strain and conditions.
- Thus, the life-cycle is simple yet efficient, and ecological success, industrial utility, contamination risk are thereby explained.
Pathogenesis of Aspergillus niger
- The infection by Aspergillus niger is usually initiated when its spores (called conidia) are inhaled into the respiratory tract, where they may get deposited deep within alveoli (the air sacs).
- Once inside, the conidia are recognized by the host immune system, but often incomplete phagocytosis happens due to various host or environmental factors, which is how colonization is begun.
- The fungus / they germinate rapidly under humid and warm (around 37°C–42 °C) conditions, producing germ tubes that elongate into hyphae; the process is enhanced in immunocompromised hosts.
- When germination proceeds, hyphal penetration of epithelial tissues occurs; this stage is critical since tissue barriers are breached and nutrients are accessed directly from host cells.
- During this phase, a variety of hydrolytic enzymes (proteases, lipases, pectinases etc.) are secreted, they degrade cellular macromolecules and extracellular matrices, leading to necrotic lesions.
- Walking through host tissues, the hyphae were observed forming dense mats which block smaller blood vessels – leading to vascular invasion and thrombosis.
- Such obstruction of capillaries results in tissue ischemia, and due to that, localized necrosis and hemorrhagic areas are frequently produced, especially in lungs/sinuses.
- The pathogen releases toxic metabolites (like oxalic acid, gliotoxin, etc.), and these compounds chelate calcium, damaging endothelial layers, while immune cells (mainly neutrophils) are suppressed.
- In some cases, oxalic acid reacts with host calcium forming calcium oxalate crystals, which have been seen deposited in tissues; they worsen inflammation and tissue injury.
- Meanwhile, oxidative stress within host cells increases; ROS (reactive oxygen species) accumulation favors fungal survival, because antioxidant defenses are partly inhibited.
- The organism / it resists immune clearance by producing melanin pigments in conidial walls – these dark pigments prevent recognition by macrophages and neutralize oxidative bursts.
- A chronic stage may develop when A. niger establishes biofilms on mucosal surfaces or implanted medical devices (catheters, prosthetics), from which continuous infection may arise.
- Host factors (such as diabetic ketoacidosis, neutropenia or corticosteroid use) enhance susceptibility; the fungus exploits reduced immunity and gains systemic dissemination.
- Disseminated aspergillosis caused by A. niger often involves secondary infection in organs like brain, kidney, heart – although pulmonary form remains most prevalent.
- In experimental models, it was shown that adhesion to epithelial cells is mediated by fungal cell wall proteins (like galactomannan components), which interact nonspecifically with host glycoproteins.
- After colonization, immune evasion occurs through down-regulation of pattern-recognition receptor signaling – mainly TLR2/TLR4, leading to delayed inflammatory response.
- Such as, in immunosuppressed animals, fungal burden in lungs increases 3–4 fold, and cytokine imbalance is evident (IL-1β reduced / TNF-α elevated).
- The overall pathogenesis process can therefore be divided into 3 overlapping phases: (i) spore germination and adhesion, (ii) tissue invasion and damage, and (iii) immune evasion & systemic spread.
- And that’s how infection of A. niger progresses, though in healthy hosts, it often remains non-invasive or just colonizing surface layers of respiratory or ear canal.
- Sometimes, the list of symptoms are variable – allergic, saprophytic or invasive forms may occur, depending mainly upon host immune state and fungal load, which is quite unpredictable.
Host immune response to Aspergillus niger
Upon inhalation, conidia of Aspergillus niger are deposited in alveoli and are recognized by innate receptors (PRRs such as TLR2, dectin-1, mannose receptor) at 37 °C, and initial binding is often opsonized.
Walking into the airway, conidia were trapped by mucus, and then phagocytosis was attempted by alveolar macrophages (AMs) which initiate oxidative and non-oxidative killing.
Phagocytosis is carried out mainly by macrophages (AMs), where ROS (reactive oxygen species) and lysosomal enzymes are deployed, yet some conidia survive within phagosomes and germination is enabled.
If germination proceeds, hyphae are produced that are too large for phagocytosis, and neutrophils are rapidly recruited to the site, NETs (neutrophil extracellular traps) formation, degranulation and oxidative burst being induced.
Neutrophils release NETs and they physically trap and damage hyphae while antimicrobial peptides are concentrated, this is an active, rapid defence.
Complement activation is triggered on fungal surfaces (classical / lectin / alternative), opsonization is thereby increased and phagocyte uptake is promoted via complement receptors.
Epithelial cells are induced to secrete cytokines / chemokines (IL-8, TNF-α, IL-1β) which recruit immune cells, they also produce antimicrobial peptides (defensins) at mucosal surfaces, and barrier function is modulated.
Dendritic cells are activated after antigen uptake, migration to draining lymph nodes is triggered, and antigen presentation to naïve T cells is effected, initiating adaptive responses.
CD4+ T cell differentiation is skewed by cytokine milieu toward Th1 (IFN-γ, IL-12) or Th17 (IL-17, IL-23) lineages, and a Th1-biased immunity is generally associated with fungal clearance.
Antibodies (IgG, IgA, IgE) are produced and they assist in opsonization and neutralization, clinicians often measure IgE for allergic presentations (ABPA), and serology may be supportive.
Allergic / hypersensitivity responses are driven by Th2 cytokines in susceptible hosts, eosinophilia and elevated IgE are seen, bronchiectasis and chronic symptoms can follow.
Immune evasion strategies are employed by the fungus, melanin is produced in conidial walls that mask PAMPs and reduce oxidative killing, and extracellular metabolites are secreted which modulate phagocyte function.
The network of cytokines are complex and regulatory cytokines (IL-10, TGF-β) are induced which may limit tissue damage but also favor fungal persistence.
In immunocompromised patients (neutropenia, corticosteroids, diabetes) unchecked growth is frequently observed, dissemination to other organs (brain, kidney, heart) may be facilitated.
Granulomatous inflammation and fibrosis are induced in chronic infections as host tries to wall off hyphae, collagen deposition and scarring may result.
Such as, excessive neutrophil protease release and ROS that damage host tissue may occur, contributing to pathology rather than protection.
Memory T and B cells are generated after antigen exposure, partial protection is conferred on re-exposure but antigen variability limits sterilizing immunity.
Biofilm formation on mucosa / implanted devices is promoted and persistent colonization is thereby established; they are more resistant to phagocytosis and to antifungal drugs.
Diagnostic biomarkers (cytokine signatures, galactomannan, BDG) are influenced by host response and sampling time (early vs late) affects sensitivity and interpretation.
Overall host defence against Aspergillus niger is multi-layered — innate cellular killing, complement, epithelial barriers and adaptive T/B arms are involved, and outcome is determined by host factors (age, comorbidity), fungal load, and timing of therapy.
Laboratory Diagnosis Methods of Aspergillus niger
Direct microscopy is usually performed first, where clinical specimens (sputum, BAL / bronchoalveolar lavage, tissue) are examined after KOH, Calcofluor white, PAS or GMS staining for septate hyphae with acute angle branching.
Walking into the bench, the wet mount was viewed under low and high power, showing conidia and rough-walled conidiophores typical of Aspergillus niger.
Culture on fungal media (SDA / Sabouraud dextrose agar; Czapek Dox) is done, incubations at 25–30 °C and sometimes at 37°C are used to promote colony growth, black powdery colonies are often produced.
Colony morphology is assessed macroscopically and microscopically, and conidiophore structure (vesicle shape, phialide arrangement) is examined for phenotypic identification.
Microscopic slides are mounted (lactophenol cotton blue, adhesive tape) and the spore arrangement is described, because species-level ID is aided by these features, yet misidentification can occurs.
Biochemical/physiological testing (carbon assimilation, acid production) may be applied as supplementary, these tests are slower and are used in reference labs.
Galactomannan (GM) antigen detection is performed on serum or BAL / serum samples by ELISA, a positive index is interpreted as supportive evidence of invasive disease, but false positives are possible.
(1→3)-β-D-glucan (BDG) assay is used in serum as a broad fungal screening test; specificity is limited since many fungi contribute BDG, interpretation should be cautious.
PCR (polymerase chain reaction) assays are applied to clinical specimens and, when species-specific primers are used, A. niger DNA is detected with high sensitivity; quantitative or real-time formats are common.
Nested PCR and multiplex formats are employed for increased sensitivity or simultaneous detection of several Aspergillus spp, and results are affected by sample type and storage.
In situ hybridization / PNA-FISH is performed on tissue sections to localize fungal nucleic acid, this method is used when histopathology is ambiguous and tissue invasion must be confirmed.
MALDI-TOF MS (mass spectrometry) is used on cultured isolates for rapid proteomic identification, Labs run it in tertiary centres and databases must be comprehensive for accurate ID.
Serologic tests (IgG, IgE) are measured especially in chronic or allergic presentations; raised IgE (or IgG) levels are supportive for ABPA or chronic pulmonary aspergillosis, but alone they are not definitive.
Lateral flow assays (LFA) and rapid antigen tests are being used on BAL/ serum for point-of-care screening; speed is an advantage, sensitivity varies.
Histopathology of biopsy tissue is performed with GMS / PAS stains, invasion of tissue by septate branching hyphae is demonstrated, and that finding is considered gold standard for invasive disease.
Antifungal susceptibility testing (AFST) is carried out on isolates to guide therapy, though standardized breakpoints for some Aspergillus spp are still debated, and many centres do not routinely perform it.
Mixed-method approaches are recommended: culture + GM + PCR (and histology when available) are combined, because single tests lack perfect sensitivity/specificity, the composite increases diagnostic confidence.
Care is required to distinguish colonization from true infection, since environmental contamination or airway colonization by A. niger they may yield positive culture or DNA without invasive disease.
The panel of diagnostic tests are listed above, and clinicians are advised to interpret results with clinical / radiological correlation, clinical judgment is essential.
Rapid communication of positive microscopy or culture is important, for therapy decisions.
Treatment of Aspergillus niger Infections
The treatment of infections caused by Aspergillus niger is mainly dependent on site, severity and host immune condition, though general antifungal approaches are mostly applied.
Usually, triazole antifungals are considered the first line therapy; Voriconazole is preferred due to its broad activity and favorable pharmacokinetics (PKs).
It is given orally / intravenously, the dose is adjusted as per serum concentration monitoring, though sometimes resistance is seen which complicates management.
In many cases, Itraconazole or Posaconazole may be used as alternative options, they have been effective in chronic pulmonary aspergillosis and otomycosis caused by A. niger.
Walking through clinical data, Amphotericin B (AmB) remains a second-line or salvage drug, however its nephrotoxicity and infusion reactions are major limitations.
Lipid formulations (L-AmB / ABLC) are used to reduce toxicity, especially when prolonged therapy is required or azoles can’t be tolerated.
Echinocandins (like Caspofungin, Micafungin) have limited efficacy against A. niger, though in combination therapy they may be used to achieve additive or synergistic effect.
Combination therapy is sometimes preferred, such as Voriconazole + Echinocandin, particularly in invasive cases that are not responding well to monotherapy.
Surgical intervention is often required for localized disease — sinusitis, fungal ball, or otitis externa — where debridement / removal of infected tissue improves drug penetration.
For aspergilloma, surgery may be performed if there is recurrent hemoptysis or large cavitary lesions; antifungal therapy alone is insufficient in many of such cases.
When immune suppression is present, correction of underlying condition (for example, reducing corticosteroids, improving neutrophil count) is crucial for recovery.
Antifungal susceptibility testing (AFST) is recommended because azole resistance in A. niger isolates has been increasingly documented in both clinical and environmental strains.
The duration of therapy varies; invasive aspergillosis may need 6–12 weeks, while chronic or allergic forms require longer—sometimes months (depending upon host response and radiologic findings).
For allergic bronchopulmonary aspergillosis (ABPA) caused by A. niger, corticosteroids are used to suppress hypersensitivity along with antifungal (Itraconazole).
Topical agents (Clotrimazole, Nystatin, etc.) are used in external ear infections or cutaneous forms, and cleaning of lesion area enhances effectivity.
Such as, for fungal sinusitis, irrigation with Amphotericin B solution and endoscopic clearance are usually done; systemic antifungal is added when invasion is suspected.
Therapeutic drug monitoring (TDM) is advised during azole therapy, because serum concentrations fluctuate widely and subtherapeutic levels may lead to failure.
Monitoring of liver function tests (LFTs) is also done regularly, since azoles are hepatically metabolized and adverse events can be dose dependent.
Resistance to triazoles often arises due to overuse of agricultural fungicides, and the same mutations (like Cyp51A gene alterations) have been found in clinical isolates too, which is concerning.
Supportive measures such as oxygen therapy, fluid balance, and management of comorbidities (like diabetes, renal dysfunction) are important to maintain host defense during infection.
The fungus, they sometimes persist in cavities even after treatment, so repeated follow-up (clinical / radiological) is required to detect relapse or residual colonization.
In refractory cases, newer antifungals such as Isavuconazole have been used; its broad activity spectrum and better safety make it a promising alternative though cost limits its availability.
Hyperbaric oxygen therapy has been tried experimentally in some chronic cases to improve local tissue oxygenation and drug penetration, though results are inconsistent.
Empirical antifungal therapy is avoided unless diagnosis is supported, since unnecessary drug exposure increases resistance risk and drug toxicity.
The list of therapeutic approaches are variable, and no single regimen suits all patients, therefore treatment is individualized based on infection site, host status, and species-level identification.
Prevention of recurrence is attempted by avoiding environmental exposure (dust, compost, damp areas) and by maintenance of immune competence.
And that’s generally how Aspergillus niger infections are treated—through a mixture of antifungal therapy, surgical control, and host immune restoration—though outcomes vary case to case.
Prevention and control of Aspergillus niger
- Exposure to Aspergillus niger spores is minimized by environmental controls and personal measures, and emphasis is placed on reducing dust, soil and organic debris in patient areas.
- HEPA (High Efficiency Particulate Air) filtration is recommended for high-risk units, HEPA filters remove >99.97% of 0.3 µm particles, and positive pressure rooms are preferred for transplant/oncology wards.
- During construction/renovation, dust suppression and sealed work zones are enforced near to immunocompromised patient units, and entrances are protected to limit spore spread.
- Walking through the corridor, spores were found on ledges and equipment, illustrating how easily surfaces get contaminated.
- Patients are advised to avoid gardening and compost handling when neutropenic, and clinicians provide mask guidance (N95) and practical advice.
- Relative humidity (RH) is controlled and kept below 60% to limit sporulation, dehumidifiers and improved ventilation being used, routine monitoring is suggested.
- HVAC maintenance schedules and filter replacement are mandated, replacement of filters are required even when overlooked by staff, records should be kept.
- Water-damaged materials are removed promptly, porous furnishings are avoided in patient rooms, and drying is performed (24–72 h for many materials) to prevent fungal growth.
- Contaminated instruments are sterilized by autoclaving or by high-temperature treatment ( >60 °C ) and chemical disinfection (chlorine, hydrogen peroxide vapor) is applied to noncritical surfaces.
- Environmental surveillance is performed by air and surface sampling, samples are cultured on SDA/Czapek media (SDA = Sabouraud dextrose agar) and results are interpreted with clinical correlation.
- Protective rooms for vulnerable patients are maintained as Hepa-filtered, positive pressure environments, and engineering controls are documented in policy.
- Preventive antifungal prophylaxis (eg., triazoles such as posaconazole) is considered in selected high-risk patients, but stewardship is emphasized to avoid resistance.
- Clinicians monitor neutropenic patients closely and they will start prophylaxis when prolonged neutropenia or graft-versus-host risk is identified.
- Laboratory work with cultures is performed under BSL-2 conditions, biological safety cabinets (Class II) are used for manipulations to reduce aerosolization.
- PPE (gloves, gowns, masks) is supplied and should be worn during specimen handling, training on donning/doffing being provided to reduce cross-contamination.
- Cleaning protocols are standardized, contact times and concentrations for disinfectants are specified, but misuse (sublethal exposure) may select for tolerant strains.
- Food and storage hygiene in industry and hospitals are enforced; grains, nuts and spices are stored dry and cool, aeration and moisture control (target moisture 12–14% for cereals) are practiced.
- Such as, compost and mulch are recognized hotspots for spores, immunocompromised persons are discouraged from handling these materials, and avoidance is advised during vulnerable periods.
- Air sampling data and contamination alerts are quickly communicated to infection control teams, immediate remediation actions are taken when thresholds are exceeded.
- Contaminated disposables (bandages, catheter sets) are replaced or sterilized, adhesive tapes and nebulizer parts being frequent reservoirs if neglected.
- Patients with chronic lung disease should avoid damp basements and dusty renovation sites, and they should wear masks when brief unavoidable exposure occurs.
- Maintenance of incubators, laminar flow cabinets and HVAC systems is documented, filter checks are scheduled (eg., every 3–12 months depending on risk) and logs are retained.
- Dry cleaning or adequate drying after wet cleaning is recommended since damp cleaning without drying can promote fungal growth, improper methods are common.
- Waste containing cultures or contaminated materials is autoclaved or incinerated, labeled segregation and safe disposal are required to prevent environmental seeding.
- Staff education in hand hygiene, mask use, and environmental cleaning is provided regularly, audits are carried out though compliance sometimes is variable.
- The fungus, they are not fully eradicable from environment, but layered controls (engineering, administrative, personal) are applied to lower infection risk.
- Community advice and patient education materials are distributed to at-risk groups, pamphlets, web pages and clinic counseling being used for outreach.
- Follow-up environmental monitoring and patient surveillance are advised after remediation, sampling at 7–14 days and again up to 30 days is often performed to confirm control.
Industrial Uses of Aspergillus niger
- The fungus Aspergillus niger is widely utilized in industrial biotechnology due to its strong enzyme-producing ability and tolerance to acidic pH, though growth conditions must be tightly monitored.
- During fermentation, large quantities of citric acid are produced by this species – it remains the most commercially important organic acid derived from microorganisms.
- Citric acid fermentation is carried out using carbohydrate substrates (like molasses/glucose/sucrose) under submerged conditions, and the process is optimized at 28–32 °C and low metal ion concentration.
- Walking into the fermentation plant, one can notice that A. niger is cultivated in stainless bioreactors where aeration, agitation, and nutrient ratios are strictly controlled but sometimes variable across batches.
- The organism has been used also for production of gluconic acid, which is formed through oxidation of glucose by the enzyme glucose oxidase (GOx), another product it secretes abundantly.
- Gluconic acid and its salts (calcium gluconate, sodium gluconate) are applied in food, pharma, and cleaning industries; and yields of 80–95% are achieved in optimized processes.
- The fungus, they are exploited for large-scale production of industrial enzymes: amylases, proteases, pectinases, phytases, lipases and catalases, each enzyme serving different industrial sectors.
- Pectinase from A. niger is used for juice clarification and pulp degradation in fruit processing industries, improving yield and brightness of juice.
- Amylase and glucoamylase are applied for starch conversion (saccharification) to produce glucose syrups / dextrose from corn and other grains, these enzymes are highly thermostable.
- Phytase produced by A. niger is incorporated into poultry feed formulations to release phosphate from phytate, thus improving nutrient absorption and reducing environmental phosphorus pollution.
- In textile industry, enzymes like catalase from A. niger are used to decompose residual hydrogen peroxide after bleaching, minimizing chemical consumption and water load.
- Production of tannase (tannin acyl hydrolase) by this species is done for beverage clarification (tea, coffee, wine), where tannins are hydrolyzed, enhancing flavor and clarity.
- Such as, in cocoa and instant tea industries, tannase and pectinase combinations are used simultaneously for product improvement.
- It is also used for organic acid mixtures (citric + oxalic acids) in cleaning agents and metal chelation formulations; A. niger shows high tolerance to acidic environments allowing stable productivity.
- Enzymes like lipase from A. niger are applied in detergent formulations, food flavor development, and ester synthesis in biotransformation industries.
- Strains are genetically improved by mutagenesis or recombinant techniques to enhance yields, though wild-type strains are still preferred for regulatory simplicity.
- Biotechnological processes involving A. niger are considered generally safe since it’s classified as GRAS (Generally Recognized As Safe) by the FDA, although certain strains may produce oxalic acid or mycotoxins rarely.
- The organism it also serves as a model system for studying protein secretion and metabolic engineering in filamentous fungi, due to well-characterized genome and secretion pathways.
- Industrial-scale production of β-galactosidase and invertase from A. niger is used in confectionary and dairy industries for lactose hydrolysis and sucrose inversion, respectively.
- In biofuel industries, A. niger derived enzymes assist in saccharification of lignocellulosic biomass to fermentable sugars, complementing yeasts for ethanol production.
- It contributes to bioremediation by degrading complex hydrocarbons, dyes, and heavy metals; its tolerance to toxic compounds makes it suitable for wastewater treatment.
- Spores and dried biomass are used as biofertilizers due to their ability to solubilize phosphate and release organic acids into soil, promoting plant growth.
- Production of citric acid using A. niger accounts for around 60–70% of world microbial acid output (industrial estimates vary by 5–10%), showing its global dominance.
- Solid-state fermentation (SSF) of agricultural residues by A. niger is used to produce feed enzymes, organic acids, and value-added products, cost-effectively utilizing agro-waste.
- The bioreactors used for submerged fermentation are sterilized before inoculation, but contamination still occurs sometimes leading to yield loss, as reported in plant operations.
- Extraction and purification of metabolites involve downstream processes like filtration, ion-exchange chromatography and crystallization, which are optimized differently for each product.
- They (the fungus) are also used in biosensors, where glucose oxidase from A. niger is immobilized on electrodes for glucose monitoring devices in clinical diagnostics.
- In leather and pulp industries, pectinolytic and proteolytic enzymes from A. niger are employed to soften hides and to improve paper pulp drainage quality.
- Research is being conducted to engineer A. niger for novel metabolites—such as itaconic acid and bio-based polymers, expansion of its industrial scope is ongoing.
- Overall, the industrial applications of Aspergillus niger span multiple sectors—food, pharma, textiles, bioenergy, agriculture—and its importance continues growing due to its versatility, stability, and productivity potential.
FAQ
What is Aspergillus niger?
Aspergillus niger is a filamentous fungus that is commonly found in soil, air, and plant material. It is known for its ability to produce a variety of enzymes and organic acids, which make it useful in a variety of industrial applications.
Is Aspergillus niger harmful to humans?
Aspergillus niger is generally not harmful to humans, although it can cause infections in people with weakened immune systems. It is also a common cause of otomycosis, a type of fungal ear infection.
How is Aspergillus niger diagnosed?
Aspergillus niger can be diagnosed through a variety of laboratory tests, including fungal culture, microscopy, and serological tests.
What are the symptoms of an Aspergillus niger infection?
Symptoms of an Aspergillus niger infection can vary depending on the location of the infection, but may include fever, cough, chest pain, and difficulty breathing.
How is Aspergillus niger treated?
Aspergillus niger infections are typically treated with antifungal medications, such as voriconazole or amphotericin B.
How can Aspergillus niger be prevented?
Aspergillus niger can be prevented by maintaining good hygiene and cleanliness, avoiding exposure to contaminated soil or plant material, and controlling moisture levels in indoor environments.
What are the industrial uses of Aspergillus niger?
Aspergillus niger is used in the production of citric acid, enzymes, organic acids, biofuels, and in soil remediation.
Can Aspergillus niger be used in food production?
Yes, Aspergillus niger is commonly used in food production as a source of citric acid, and as a fermentation agent in the production of certain foods, such as soy sauce and tempeh.
What is the life cycle of Aspergillus niger?
Aspergillus niger reproduces asexually by forming conidial spores. The spores germinate and form a vegetative cell, which develops into hyphal mycelium. The aerial hyphae then grow to form conidiophores, which produce conidial spores.
Where is Aspergillus niger commonly found?
Aspergillus niger is commonly found in soil, air, and plant material, and can also be found in indoor environments with high moisture levels, such as bathrooms and kitchens.
- Person AK, Chudgar SM, Norton BL, Tong BC, Stout JE. Aspergillus niger: an unusual cause of invasive pulmonary aspergillosis. J Med Microbiol. 2010 Jul;59(Pt 7):834-838. doi: 10.1099/jmm.0.018309-0. Epub 2010 Mar 18. PMID: 20299503; PMCID: PMC3052473.
- https://www.toppr.com/guides/biology/microorganisms/aspergillus-niger-and-its-uses/
- https://www.inspq.qc.ca/en/moulds/fact-sheets/aspergillus-niger
- https://www.creative-biolabs.com/drug-discovery/therapeutics/aspergillus-niger.htm
- https://www.news-medical.net/life-sciences/What-is-Aspergillus-niger.aspx
- https://en.wikipedia.org/wiki/Aspergillus_niger
- https://www.microscopemaster.com/aspergillus.html
- https://www.researchgate.net/figure/Morphological-characteristics-of-Aspergillus-niger-volumetric-power-input-100-W-m-3_fig1_268427977
- https://www.researchgate.net/publication/340398703_Aspergillus_niger_Spores_Are_Highly_Resistant_to_Space_Radiation
- https://www.ijpsonline.com/articles/fungal-biodiversity-of-a-library-and-cellulolytic-activity-of-some-fungi.html?view=mobile
- https://www.pjms.com.pk/issues/octdec207/article/article9.html
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.