What is Thyroid Gland?
- The thyroid gland is an important hormone-producing organ in the front of the neck, just below the Adam’s apple. This gland is very important for keeping the body’s metabolism running smoothly because it makes thyroid hormones like calcitonin and thyroxine (T4) and triiodothyronine (T3). These hormones are very important for keeping serum calcium levels steady, controlling metabolism, and helping cells grow and develop.
- The thyroid is small and has a lot of blood vessels, which is typical of hormonal glands. Its unique shape comes from having two side lobes linked by a thin band of tissue called the isthmus. It is situated between the 5th and 7th cervical vertebrae. Because of its unique shape, the gland can closely connect with nearby body parts, like the parathyroid glands (they keep calcium levels stable) and the recurrent laryngeal nerves (they send nerve signals to the vocal cords).
- This part of the brain controls the anterior pituitary gland’s job by releasing thyroid-stimulating hormone (TSH). The brain controls TSH by letting out thyrotropin-releasing hormone (TRH). This control system makes sure that the body’s metabolism is met by the production of thyroid hormones. The fact that the gland is the body’s biggest pure endocrine gland shows how important it is.
- It is necessary for the body to get iodine from food in order to make thyroid hormones T4 and T3. These hormones impact a multitude of physiological processes, including fat metabolism, thermogenesis, and growth and development, especially in children. Their amounts need to be carefully controlled. Too much can cause hyperthyroidism, which is marked by a fast metabolism, and not enough can cause hypothyroidism, which is marked by a slow metabolism.
- The thyroid gland is also involved in a number of diseases, such as autoimmune diseases like Graves’ disease and inflammatory diseases like thyroiditis. The production of hormones can become very faulty in these situations. Furthermore, the thyroid gland is susceptible to various types of cancer, including papillary, medullary, and follicular carcinoma, each needing different methods to detection and treatment.
- Calcitonin is another hormone that the thyroid makes and is very important for breaking down calcium. It lowers calcium levels in the blood by stopping osteoclasts from breaking down bone cells. This stops calcium from entering the bloodstream. This hormone, along with parathyroid hormone (PTH), helps to keep calcium homeostasis, an important aspect of different bodily processes.
Definition of Thyroid Gland
The thyroid gland is a butterfly-shaped endocrine organ located in the anterior neck that produces hormones such as thyroxine (T4), triiodothyronine (T3), and calcitonin, which regulate metabolism, growth, and calcium homeostasis. It plays a crucial role in maintaining the body’s metabolic rate and overall hormonal balance.
Anatomical Location of Thyroid Gland
Understanding the interaction of the thyroid gland with surrounding tissues and its functional consequences depends on its anatomical location. Located in the front of the neck, this gland is central and helps greatly in many physiological processes.
- Location: From the fifth cervical vertebra (C5) to the first thoracic vertebra (T1), the thyroid gland runs in the front neck.
- The gland has a distinct butterfly form as its two lateral lobes—left and right—are linked by a central isthmus. Its orientation and purpose are made easier by this special form.
- The thyroid’s lobes are tightly wrapped around the superior rings of the trachea and the cricoid cartilage. This intimate relationship helps the thyroid gland to connect with the airway and affects its operation in control of metabolism and other body functions.
- Visceral Compartment: The thyroid gland is positioned within the visceral compartment of the neck. Important structures including the trachea, esophagus, and throat also fall under this section. Pretracheal fascia encloses the visceral compartment and offers structural support while defining the neck’s space.
- Anatomical Relationships: Surgical operations depend much on the thyroid gland’s anatomical orientation. Surgical treatments involving the thyroid can be difficult given its close proximity to significant structures including the parathyroid glands and the recurrent laryngeal nerves.
- Third or fourth week of pregnancy marks the thyroid gland’s embryological development from the pharynx. It moves gradually down to reach its lowest point at the base of the neck.
- Understanding the anatomical location is crucial for both planning surgical techniques to prevent damage to surrounding vital structures and in diagnosis and treatment of thyroid diseases.
Structure of Thyroid Gland
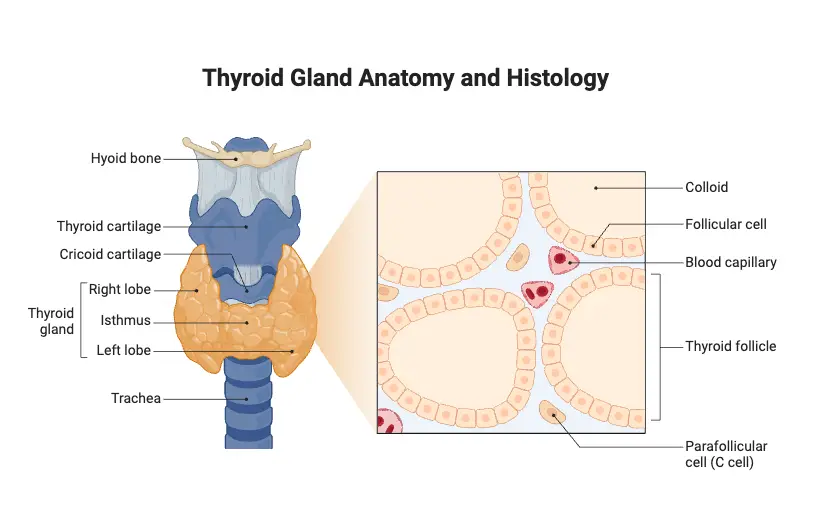
- Present on the anterior side of the neck, in front of the trachea, the thyroid gland is butterfly-shaped.
- Comprising two lobes on either side of the trachea, the gland weights around 25 grams. Every lobe in the lobes is cone-shaped, five-centimeter length, three-centimeter width.
- The isthmus, a middle mass of tissue, ties the two lobes together in between them.
- Hollow spherical follicles that remain scattered over the gland’s internal structure make up its architecture.
- Cuboidal epithelial cells—also called follicular cells—make up the walls of these follicles.
- Thyroid hormones produced by these cells are glycoprotein known as thyroglobulin. The hormone comes out as a sticky, amber-colored liquid known as colloid.
- Apart from the follicular cells, the thyroid gland consists in parafollicular cells that generate calcitonin.
- Present inside the follicular epithelium, the parafollicular cells do not develop separately with the connective tissue.
- Supplied with arterial blood flow via the superior and inferior thyroid arteries, the thyroid gland is rather vascular.
Hormones of Thyroid Gland
The thyroid gland is an essential component of the endocrine system, producing hormones that significantly influence various physiological processes. The primary hormones secreted by the thyroid gland include thyroxine (T4), triiodothyronine (T3), and calcitonin. Each of these hormones plays a crucial role in regulating metabolism and calcium levels in the body.
- Thyroid Hormones: The thyroid hormones consist mainly of thyroxine (T4) and triiodothyronine (T3).
- Thyroxine (T4):
- Thyroxine contains four iodine atoms and is created by the follicular cells of the thyroid gland.
- Except for the lungs, brain, testis, and retina, most tissues depend on this hormone to boost their oxygen consumption, so increasing their basal metabolic rate (BMR).
- Especially in youngsters, the development of skeletal tissues depends on it.
- Thyroxine controls carbohydrate metabolism and encourages gluconeogenesis—the synthesis of glucose from non-carbohydrates.
- Furthermore affecting lipid metabolism and increasing sodium-potassium ATPase activity is important for preserving cellular ion balance.
- Furthermore helping the body to control water and electrolyte balance is thyroxine.
- The production of T4 is primarily regulated by thyroid-stimulating hormone (TSH) released from the pituitary gland.
- In peripheral tissues, T4 is turned into T3; T3 is around five times more strong than T4 in inducing metabolic activity.
- Deficiencies in thyroxine can lead to illnesses such as goiter and hypothyroidism, whereas excess levels can result in thyrotoxicosis and hyperthyroidism.
- Triiodothyronine (T3):
- Triiodothyronine consists of three iodine atoms and is predominantly produced via the conversion of T4.
- More importantly for controlling metabolic activity than T4, T3 affects target tissues more broadly.
- Thyroxine (T4):
- Calcitonin:
- Made by parafollicular cells (C cells) of the thyroid gland, calcitonin is a polypeptide hormone.
- Its release is predominantly driven by high calcium levels in the circulation, rather than by feedback processes involving the pituitary gland.
- The major function of calcitonin is to reduce blood calcium levels. This is accomplished by responding antagonistically to parathyroid hormone (PTH).
- Calcitonin lowers osteoclastic activity, which decreases the release of calcium from bones into the circulation.
- Additionally, calcitonin increases the transport of calcium from the blood to the bone matrix, so assisting in bone growth and mineralization.
- Particularly in treating osteoporosis, where it acts as a bone-sparing agent, this hormone has drawn interest for its therapeutic value.
Some of the essential functions of the thyroid hormones
Thyroid hormones, especially thyroxine (T4) and triiodothyronine (T3), are very important to how the body works. These hormones, which are made by the thyroid gland, are very important for keeping homeostasis and affecting many biological processes. The most important things that thyroid hormones do are listed below:
- Development and Growth: Hormones from the thyroid are important for the development, growth, and division of almost all body cells. During childhood and puberty, they are especially important because they help with general physical and mental development.
- Controlling the Basal Metabolic Rate (BMR): These hormones play a big role in controlling BMR, which is the rate at which the body burns calories when it’s not doing anything. When amounts of thyroid hormones are high, BMR usually goes up, which makes the body use more energy.
- How Calcium Is Used: Hormones in the thyroid help keep calcium levels in check in the body. By helping calcium and phosphate build up in the bones, they make the bones stronger and denser. They also help lower serum calcium levels by making bones store calcium.
- Development of the Central Nervous System (CNS): Thyroid hormones are very important for the development and proper working of the CNS in children. They have an effect on neurogenesis, synaptogenesis, and myelination, all of which are important for brain health and cognitive performance.
- Both physical and mental growth are sped up by thyroid hormones. This can have an effect on mood, thinking, and general mental health.
- Hormones have a direct effect on cardiovascular physiology. They raise heart rate and make heart contractions stronger, which increases cardiac output.
- Metabolism of Macromolecules: Hormones from the thyroid are very important for breaking down carbohydrates, fats, and proteins. They improve the breakdown of these macromolecules, which makes it easier for the body to make and use energy.
- Vitamin Metabolism: Hormones also affect vitamin metabolism, which makes sure that the body gets all the nutrients it needs quickly.
- Controlling body temperature: Thyroid hormones play a big part in controlling body temperature by managing biological processes. They help the body make more heat, which is especially important when it’s cold outside.
- Metabolism of Lipids: Thyroid hormones help break down cholesterol and fats, which changes lipid levels and metabolic health in general.
- Balance of Electrolytes: They help keep the balance of electrolytes, which is important for cells to work normally and for keeping health in the body as a whole.
- erythropoiesis: Thyroid hormones help make red blood cells (RBCs) by increasing the production of erythropoietin and improving the body’s iron absorption.
- Activity in the mitochondria: These hormones improve mitochondrial metabolism, which makes cells breathe and make more energy. As a result, they make cells and organs use more oxygen.
- Behaviour and Mood: Thyroid hormone levels and mood regulation are somewhat connected. Mood disorders like depression and anxiety can be caused by levels that aren’t normal.
- Gut Movement: Thyroid hormones make the intestines move, which makes digestion and nutrient absorption easier.
- Beta-Adrenergic Sensitivity: They make beta-adrenergic receptors more sensitive to catecholamines. This can help the body respond better to hormonal and stress messages.
Embryology and histology of Thyroid Gland
The thyroid gland is a critical endocrine organ that undergoes complex developmental processes during embryogenesis and exhibits distinctive histological features. Understanding the embryology and histology of the thyroid gland is essential for grasping its function and potential pathologies.
- Embryology:
- During the third gestational week of development, the thyroid gland begins life in the endodermal layer. It results especially from the junction of the base of the tongue along the median line with the fourth pharyngeal pouch.
- The growing fetus starts using iodine by the tenth week of pregnancy since it is necessary for synthesis of thyroid hormones. Fetal blood at this point shows detectable quantities of thyroid-stimulating hormone (TSH) and thyroxine (T4).
- Particularly T4, the production of thyroid hormones rises in the second trimester to reflect the growth of the fetal thyroid gland. At the same time, the hypothalamus grows and is essential for the synthesis of thyrotropin-releasing hormone (TRH), therefore triggering TSH generation.
- It is noteworthy that TSH does not traverse the placenta from mother to fetus although TRH may. Maternal hormone levels so largely determine the activity of the fetal thyroid gland.
- Beginning at the end of the second trimester, triiodothyroid (T3) levels start to rise; their synthesis increases after delivery.
- Key transcription factors control the development of the thyroid gland: paired homeoboxin-8 (PAX-8), thyroid transcription factor 1 (TTF-1 or NKX2A), and thyroid transcription factor 2 (TTF-2). Follicular cell development as well as the expression of thyroid-specific proteins—including thyroid-specific TSH receptor and thyroglobulin—depend on these elements.
- Because of thyroid agenesis or reduced hormone output, mutations in these transcription factors may cause congenital hypothyroidism.
- Histology:
- The basic structural unit of the thyroid gland is the thyroid follicle, whose diameter falls between 100 and 300 micrometers. Each follicle includes a single layer of follicular cells around a central lumen filled with colloid.
- Synthesizing thyroid hormone precursors, follicular cells secrete thyroglobulin into the colloidal lumen. Thyroid hormones find storage in this colloid.
- The follicular cells’ apical surface contacts the colloid, which helps iodide be absorbed and T3 and T4 to be synthesized later on. Richly vascularized, basic nature of these cells enables effective hormonal release into the circulation.
- Furthermore present in the thyroid gland are parafollicular cells, sometimes known as C-cells, which secrete calcitonin. This hormone has a function in controlling calcium homeostasis in the body by lowering blood calcium levels via its effects on bone metabolism.
Thyroid hormone synthesis
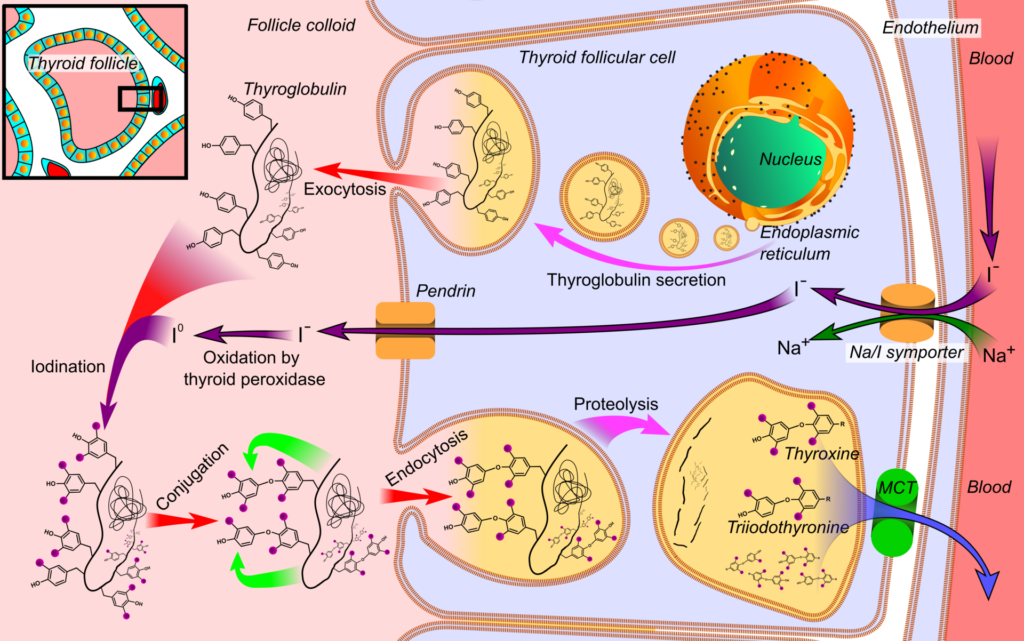
Essential for control of metabolism and development in the human body, thyroid hormone production is a complicated biochemical process taking place within the thyroid gland. Thyroxine (T4) and triiodothyronine (T3) are the main hormones generated; T4 is the main type synthesised whereas T3 is more physiologically active. There are four separate phases to the synthesis of these hormones:
- Iodine Uptake
- Iodine is first actively transported into thyroid follicular cells via the sodium-iodide symporter (Na+/I-symporter).
- Thyroid-stimulating hormone (TSH) is mostly responsible for this transport system activation. The absorption of iodine is susceptible to concentration-dependent autocontrol, where higher intracellular iodine levels prevent further transport.
- Different anions, including perchlorate, pertechnetate, and thiocyanate, might interfere with this iodine movement, hence stressing an important phase of the synthesis process.
- Iodine Oxidation
- The second stage is typified by the oxidation of iodide to iodine, a reaction made possible by the enzyme thyroperoxidase, which requires hydrogen peroxide (H2O2) as a cofactor.
- This reaction takes place in the follicular lumen; the oxidation process is essential for preparing iodine for later binding to tyrosine residues in thyroid lumbine. Some drugs, notably methimazole and propylthiouracil, might block this enzymatic pathway, hence influencing hormone production.
- Iodination of Tyrosine
- The third step is organification—that is, the iodination of tyrosine residues found in thyroglobulin.
- Iodine hooks to tyrosine at this step to generate two crucial intermediates: diiodotyrosine (DIT) and monoiodotyrosine (MIT). These are regarded as dormant versions of thyroid hormones.
- Thyroid gland protein content is around 70% composed of thyroglobulin, a glycoprotein with a molecular weight of roughly 660 kDa that incorporates several tyrosine residues.
- The third step is organification—that is, the iodination of tyrosine residues found in thyroglobulin.
- Coupling Reactions:
- The coupling of MIT and DIT generates the active thyroid hormones in the last step of synthesis:
- MIT + DIT → T3
- DIT + DIT → T4
- Furthermore stressing the interdependence of these processes, T3 may also be produced from the metabolism of T4 in peripheral organs.
- The coupling of MIT and DIT generates the active thyroid hormones in the last step of synthesis:
Transported to the apical membrane of the follicular cells and secreted into the follicular lumen by exocytosis, the synthetic thyroid, with notable levels of MIT, DIT, T3, and T4 residues, is The next release of T3 and T4 into circulation depends on this storage form, hence it is absolutely essential.
Thyroid hormone secretion
The thyroid gland secretes T3 (triiodothyronine) and T4 (thyroxine) into the circulation in a carefully controlled mechanism. These hormones control metabolic homeostasis and other physiological functions. Several phases comprise the process:
- Follicular cells generate and store thyroid hormones linked to thyroglobulin glycoprotein. This hormone storage mechanism ensures a ready supply.
- The anterior pituitary gland releases thyroid-stimulating hormone (TSH) to stimulate thyroid hormone output. Follicular cells have more apical microvilli when TSH levels rise.
- Upon TSH activation, follicular cells participate in pinocytosis, engulfing colloid droplets carrying thyroglobulin and thyroid hormones. Microtubules help return droplets to the follicular cell’s apex.
- When colloidal pinocytic vesicles reach the cytoplasm, they merge with lysosomes, becoming fagolysosomes. This fusion starts thyroglobulin hydrolysis.
- Thyroglobulin hydrolysis occurs in fagolysosomes via lysosomal proteases. T3 and T4 are made from tyrosine residues released by this enzymatic breakdown.
- Thyroid hormones (T3 and T4) are released from follicular cells into the circulation by diffusion. This process delivers hormones quickly to target tissues for metabolic effects.
- Not all iodinated chemicals from hydrolysis are discharged into circulation. Monoiodotyrosine (MIT) and diiodotyrosine (DIT) are not secreted and are instead recycled by deiodination.
- The enzyme dehalogenase removes iodine atoms from T4 to create T3 in this recycling process.
- Up to 50% of the iodine in thyroglobulin may be reused by deiodination processes. Maintaining iodine levels and avoiding shortages requires this technique.
- Deficient deiodinase enzymes may cause iodine shortage and hypothyroid goiter. Patients may need iodine supplementation to restore thyroid function.
Thyroid hormone transport
Thyroid hormone transport is a crucial physiological process that regulates the distribution and availability of T3 (triiodothyronine) and T4 (thyroxine) hormones in the bloodstream. Upon their release from the thyroid gland, these hormones bind to specific carrier proteins synthesized primarily in the liver, which play a significant role in maintaining hormonal balance. The transport mechanism can be outlined as follows:
- Binding to Carrier Proteins:
- Once thyroid hormones enter circulation, they predominantly bind to plasma proteins, which renders them inactive. This binding mechanism serves a dual purpose: it reduces the excretion of hormones through urine and acts as a reservoir to modulate hormone availability in the bloodstream.
- Main Carrier Proteins:
- The primary carrier proteins involved in thyroid hormone transport include:
- Thyroxine-Binding Globulin (TBG):
- TBG is the most significant transport protein, with a molecular weight of 54 kDa.
- Although its plasma concentration is relatively low, it binds approximately 75% of circulating T4.
- TBG facilitates the diffusion of T4 into extracellular fluids, and while its levels can increase total T3 and T4, it does not influence the levels of free T3 and T4.
- Thyroxine-Binding Prealbumin (Transthyretin, TTR):
- TTR has a molecular weight of 55 kDa and exhibits lower binding affinity compared to TBG.
- It is present in plasma at about 1/100th the concentration of TBG and binds thyroid hormones at a reduced rate.
- Serum Albumin:
- With a molecular weight of 65 kDa, serum albumin is the most abundant plasma protein, but it binds thyroid hormones less effectively.
- Thyroxine-Binding Globulin (TBG):
- The primary carrier proteins involved in thyroid hormone transport include:
- Differential Binding and Activity:
- T3 binds to carrier proteins in significantly lower amounts compared to T4. Consequently, T3 remains more active in the intracellular environment.
- The affinity of carrier proteins for T4 means that when T4 enters cells, it typically binds to cytoplasmic proteins that facilitate its conversion into the more active T3.
- Half-Life of Thyroid Hormones:
- The half-life of T4 in circulation is approximately six days, allowing for a steady release of T4 to maintain physiological functions.
- In contrast, T3 has a much shorter half-life of less than one day, which aligns with its higher activity level in cellular processes.
- Physiological Implications:
- The reversible binding of thyroid hormones to carrier proteins ensures that the free, biologically active form of these hormones is maintained at levels necessary for metabolic regulation. This dynamic equilibrium allows for rapid hormonal responses to physiological demands while preventing excess hormone levels that could lead to pathological conditions.
Diseases and Disorders of Thyroid Gland
Diseases and disorders of the thyroid gland encompass a wide range of conditions that significantly impact metabolic processes and overall health. The thyroid gland, responsible for producing essential hormones, can develop various abnormalities, each with distinct etiologies and manifestations. Below is an overview of the key diseases and disorders associated with the thyroid gland:
- Goiter: Goiter refers to an abnormal enlargement of the thyroid gland. It can be categorized into several types:
- Uni-nodular Goiter: Characterized by a single enlarged nodule in the thyroid gland.
- Multinodular Goiter: Involves multiple nodules and is often associated with varying hormone levels.
- Diffuse Goiter: Represents uniform enlargement of the thyroid without discrete nodules.
- Colloid Nodular Goiter: The most common non-neoplastic lesion, where thyroid follicles are filled with colloid and lined by squamous follicular cells.
- Hyperthyroidism (Thyrotoxicosis): This condition is marked by an excessive secretion of thyroid hormones, leading to a hypermetabolic state. Increased levels of T3 and T4 result in symptoms such as:
- Palpitations and tachycardia
- Nervousness and anxiety
- Increased sweating and weight loss
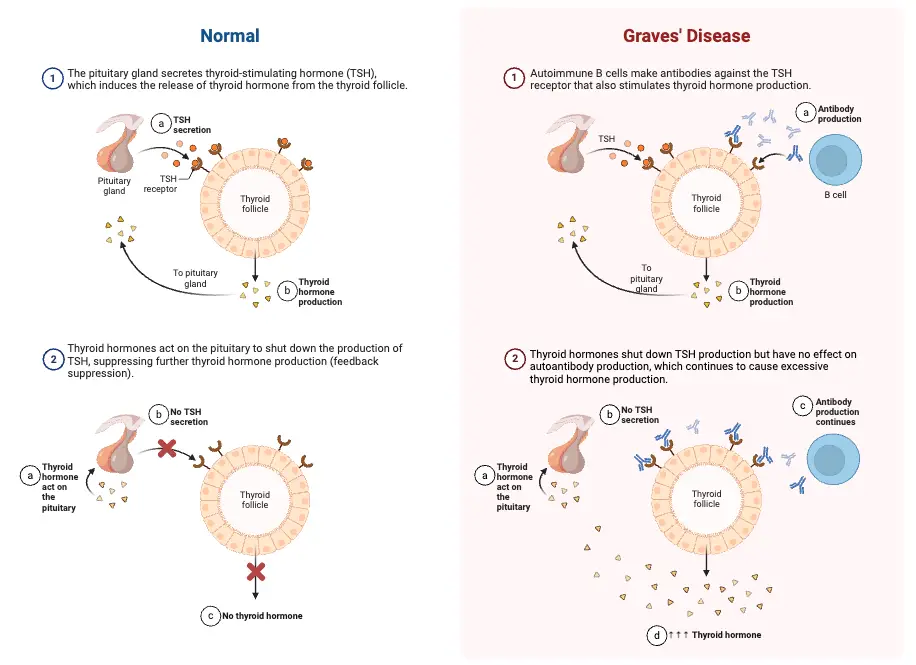
- Graves’ Disease: An autoimmune disorder primarily affecting women aged 30 to 50, Graves’ disease is characterized by the hypersecretion of thyroid hormones. The immune system produces antibodies that mimic thyroid-stimulating hormone (TSH), resulting in:
- Prolonged palpitations
- Exophthalmos (protrusion of the eyeballs)
- Dermopathy (myxedema)
- Increased metabolic activity, which may lead to weight loss and heat intolerance
- Hypothyroidism: This condition arises from diminished production of thyroid hormones due to various factors, including autoimmune disorders, iodine deficiency, or thyroiditis. It manifests differently in individuals:
- Cretinism: A severe form of hypothyroidism in infants characterized by physical and mental retardation, stunted growth, and developmental delays. This can result from maternal iodine deficiency or genetic factors affecting thyroid function.
- Myxedema: Occurring in adults, this condition presents with symptoms such as:
- Puffiness of the skin
- Low body temperature and bradycardia
- Reduced basal metabolic rate (BMR)
- Mental sluggishness and fatigue
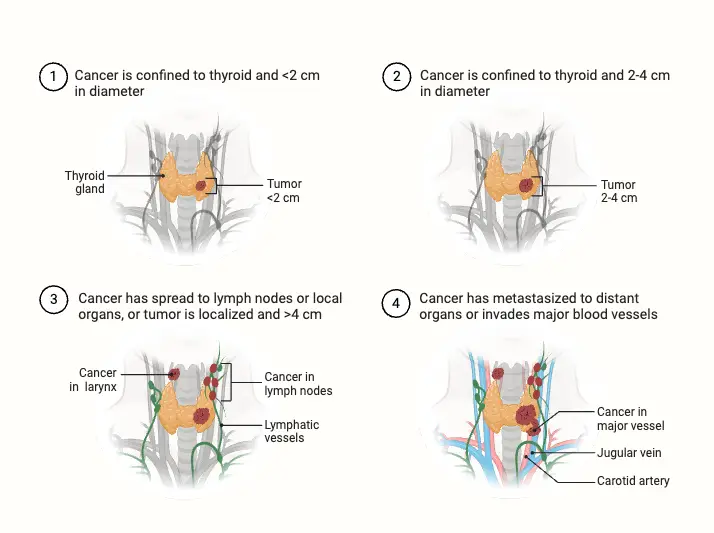
- Thyroid Cancer: Thyroid carcinomas can originate from either follicular epithelium or parafollicular C-cells, often presenting as painless nodules. Types of thyroid cancer include:
- Papillary Carcinoma: The most common form, typically slow-growing and highly treatable.
- Follicular Carcinoma: More aggressive than papillary carcinoma, can metastasize to distant organs.
- Anaplastic Carcinoma: A rare and aggressive form with a poor prognosis.
- Medullary Carcinoma: Arising from C-cells, often associated with multiple endocrine neoplasia (MEN) syndromes.
- Thyroid Tumors: These can be benign or malignant. Benign adenomas are relatively common, while malignancies are more frequent in older adults. Some benign tumors may secrete excess thyroid hormones, leading to hyperthyroidism symptoms. Regular monitoring, including imaging studies, is essential for determining the tumor’s nature and guiding treatment.
- Exophthalmic Goiter: Closely linked with Graves’ disease, exophthalmic goiter involves protrusion of the eyeballs (exophthalmos) and is accompanied by symptoms of hyperthyroidism. This condition highlights the systemic effects of excessive thyroid hormones, particularly on ocular health and overall physiology.
Effects of thyroid hormones
Thyroid hormones, primarily thyroxine (T4) and triiodothyronine (T3), exert a broad range of effects on cellular functions, growth, metabolism, and various physiological systems. Understanding these effects is crucial for students and educators alike, as thyroid hormones play a pivotal role in maintaining homeostasis and influencing overall health.
- Cellular Effects:
- At the cellular level, T3 facilitates the synthesis of proteins, including enzymes, through transcription and translation processes. These proteins are produced after T3 interacts with specific receptors in the nucleus.
- An increase in protein synthesis is accompanied by a rise in catabolism, significantly boosting the basal metabolic rate, which can escalate by 60-100% in instances of excessive thyroid hormone secretion.
- Thyroid hormones enhance mitochondrial activity, increasing mRNA synthesis and protein production, particularly respiratory chain proteins. This stimulation leads to enhanced ATP synthesis and oxygen consumption, indicating a direct correlation between thyroid hormones and cellular energy metabolism.
- The production of transport enzymes, such as the Na⁺-K⁺-ATPase pump, increases due to the heightened protein synthesis. This pump regulates sodium and potassium ion concentrations across cell membranes, which further accelerates metabolic processes.
- The Ca²⁺-ATPase enzyme also plays a crucial role in maintaining calcium ion homeostasis, particularly in muscle contractions and neurotransmitter release.
- Effects on Growth:
- Thyroid hormones are essential for normal growth and development, influencing both specific and general growth processes. In children, hypothyroidism often results in stunted growth due to premature closure of the epiphyses, while hyperthyroid children may exhibit increased height.
- These hormones are also critical for brain development during both pre- and post-natal periods. A deficiency in maternal thyroid hormone can lead to significant developmental delays and neurological impairments in the fetus.
- Normal serum levels of T4 (5-12 µg/dL) and T3 (80-200 ng/dL) are crucial for ensuring appropriate growth; early detection and treatment of thyroid hormone deficiencies in newborns can lead to normal development.
- Metabolic Effects:
- Thyroid hormones regulate carbohydrate metabolism by promoting both anabolic and catabolic pathways. They enhance the synthesis of enzymes involved in carbohydrate metabolism, thereby increasing glucose uptake by cells and stimulating both glycolysis and gluconeogenesis.
- In lipid metabolism, thyroid hormones also exhibit both anabolic and catabolic properties. They stimulate lipolysis in adipose tissue, resulting in elevated free fatty acid concentrations in the bloodstream. Notably, while cholesterol and triglyceride levels are expected to rise, they often remain low due to the increased receptor synthesis for LDL and cholesterol in the liver, facilitating their clearance from circulation.
- Additionally, thyroid hormones enhance protein metabolism by promoting amino acid transport and increasing protein synthesis. This anabolic effect supports normal growth and development, particularly in fetal stages, by synthesizing insulin-like growth factors.
- Effects on Physiological Systems:
- In the cardiovascular system, thyroid hormones primarily exert their effects through the modulation of catecholamines. They increase the number of β-adrenergic receptors, which results in elevated heart rate, stroke volume, and cardiac output. In hyperthyroidism, this can lead to warm, moist skin and symptoms of restlessness due to heightened sympathetic activity.
- In contrast, hypothyroidism leads to a reduction in β-adrenergic receptor expression, resulting in decreased heart rate and cold, dry skin. Studies have shown that cardiac parameters significantly fluctuate between hyperthyroid and hypothyroid patients.
- Respiratory effects are also evident, as increased metabolic activity leads to heightened oxygen consumption and carbon dioxide production, prompting hyperventilation.
- Gastrointestinal activity is stimulated by thyroid hormones, resulting in increased appetite and digestive processes. Conversely, excessive thyroid hormone can lead to diarrhea, while hypothyroidism is often associated with constipation.
- In terms of skeletal effects, thyroid hormones influence bone metabolism by stimulating the activity of both osteoblasts and osteoclasts. While they promote bone growth in normal physiology, excessive levels can lead to increased bone resorption, thereby heightening the risk of osteoporosis, particularly in postmenopausal women.
- Moreover, thyroid hormones impact the central nervous system, influencing muscle tone and neuromuscular coordination. Symptoms such as muscle tremors and fatigue are common in hyperthyroid individuals, while hypothyroidism may lead to lethargy and increased sleepiness.
- Finally, thyroid hormones facilitate erythropoiesis by stimulating erythroid stem cells in response to oxygen deprivation. They increase erythropoietin production in the kidneys, further promoting red blood cell synthesis, which is often diminished in hypothyroid patients.
6 Steps of Thyroid Hormone Synthesis
Thyroid hormone synthesis is a complex biochemical process essential for the production of the hormones thyroxine (T4) and triiodothyronine (T3). These hormones play critical roles in regulating metabolism, growth, and development. The synthesis process occurs within the thyroid follicular cells and can be delineated into six key steps, which can be remembered using the mnemonic “ATE ICE.”
- Active Transport of Iodide:
- The process begins with the active transport of iodide into the follicular cells. This is facilitated by the sodium-iodide symporter (NIS), which utilizes a sodium gradient established by the sodium-potassium ATPase.
- The transport of iodide is crucial because it provides the necessary substrate for thyroid hormone synthesis.
- Thyroglobulin Formation:
- Within the ribosomes of the follicular cells, thyroglobulin (Tg) is synthesized. This large protein is rich in the amino acid tyrosine and serves as the precursor for thyroid hormones.
- After its formation, thyroglobulin is packaged into secretory vesicles for transport to the follicle lumen.
- Exocytosis of Thyroglobulin:
- The secretory vesicles undergo exocytosis, releasing thyroglobulin into the lumen of the thyroid follicle, where it is stored as colloid.
- This colloid serves as a scaffold for subsequent hormone synthesis, enabling the iodination and coupling processes that follow.
- Iodination of Thyroglobulin:
- In the follicle lumen, iodide becomes reactive through the action of the enzyme thyroid peroxidase.
- Iodide atoms attach to the tyrosine residues within the thyroglobulin molecule, resulting in the formation of monoiodotyrosine (MIT) and diiodotyrosine (DIT).
- Coupling Reactions:
- The next critical step involves the coupling of MIT and DIT, which generates the active hormone triiodothyronine (T3).
- Additionally, coupling two DIT molecules results in the synthesis of thyroxine (T4), the primary form of thyroid hormone released into circulation.
- Endocytosis and Proteolysis:
- The final step is the endocytosis of iodinated thyroglobulin back into the follicular cells.
- Within lysosomes, thyroglobulin undergoes proteolysis, whereby iodinated tyrosine residues are cleaved from the protein scaffold. This process liberates the free T3 and T4 hormones for release into the bloodstream.
Both T3 and T4 are lipophilic hormones predominantly transported in the blood bound to plasma proteins, such as thyronine-binding globulin (TBG) and albumin. While T3 is biologically more potent than T4, it has a shorter half-life due to a lower affinity for these binding proteins, with less than 1% of T3 and T4 remaining in an unbound free form.
In peripheral tissues, T4 can be converted into T3 through deiodination, enhancing its metabolic effects. Moreover, both T3 and T4 can be inactivated by the removal of iodine, primarily in the liver and kidneys. Due to its longer half-life, T4 is often preferred for therapeutic purposes in treating hypothyroidism, as it allows for more stable plasma concentrations compared to T3.
Hypothalamus-hypophysis-thyroid axe
The hypothalamus-hypophysis-thyroid axis (HPT axis) regulates thyroid hormone synthesis and secretion through a feedback loop involving the hypothalamus, the pituitary gland (hypophysis), and the thyroid gland. This system ensures the proper levels of thyroid hormones necessary for metabolic and physiological functions.
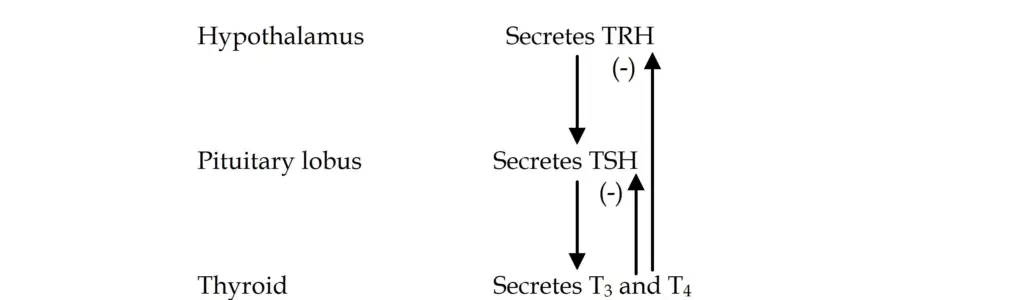
- Synthesis of Thyrotropin-Releasing Hormone (TRH):
- The process begins in the hypothalamus, where thyrotropin-releasing hormone (TRH) is synthesized in the periventricular nucleus. TRH is a tripeptide hormone and plays a pivotal role in stimulating the release of thyroid-stimulating hormone (TSH) from the anterior pituitary.
- TRH is transported from the hypothalamus to the anterior pituitary via the hypophyseal portal circulation. Upon reaching the thyrotrope cells of the anterior pituitary, TRH binds to specific receptors on the surface of these cells.
- Stimulation of TSH Secretion:
- The interaction of TRH with its receptors activates Gq proteins, which then trigger phospholipase C. This enzyme cleaves membrane phospholipids into diacylglycerol (DAG) and inositol triphosphate (IP3), both of which serve as secondary messengers.
- IP3 stimulates the release of calcium ions from the endoplasmic reticulum, while DAG activates protein kinase C. Together, these signals facilitate the secretion of TSH from the anterior pituitary into the bloodstream.
- TSH Structure and Activation:
- TSH is a glycoprotein hormone composed of two subunits: alpha (α) and beta (β). While the α subunit is shared with other hormones like luteinizing hormone (LH) and follicle-stimulating hormone (FSH), the β subunit is unique to TSH and determines its receptor specificity.
- Once released into circulation, TSH binds to TSH receptors on the thyroid gland’s follicular cells, where it activates a Gs protein that stimulates adenylate cyclase. This, in turn, increases cyclic AMP (cAMP) levels, which play a key role in promoting thyroid hormone synthesis.
- Thyroid Hormone Production and Feedback Mechanism:
- TSH stimulates the thyroid gland to synthesize and secrete thyroxine (T4) and triiodothyronine (T3), essential hormones for regulating metabolism. The production of T3 and T4 depends on the availability of iodide and the activity of thyroid peroxidase.
- However, if circulating levels of T3 and T4 become too high, they exert negative feedback on both the hypothalamus and the pituitary gland. This feedback loop inhibits the secretion of TRH from the hypothalamus and TSH from the pituitary, thereby reducing further thyroid hormone production.
- Circadian Rhythm and TSH Regulation:
- TSH secretion is pulsatile and follows a circadian rhythm, with its highest levels occurring at night and peaking around midnight. Throughout the day, TSH levels gradually decline, reflecting the body’s changing metabolic needs.
- Besides TRH, TSH secretion is influenced by other factors. Estrogen can enhance TSH secretion, while somatostatin, dopamine, and glucocorticoids suppress it. Additionally, T3 and T4 themselves inhibit TSH release through feedback regulation.
- Effects of TSH:
- The actions of TSH on the thyroid gland can be divided into three time-dependent effects:
- Immediate Effects: TSH rapidly stimulates the synthesis of thyroid hormones within minutes.
- Intermediate Effects: Over the course of hours, TSH promotes the growth of thyroid follicular cells and enhances their ability to produce hormones.
- Chronic Effects: Long-term exposure to elevated TSH levels leads to thyroid hypertrophy and hyperplasia, which can result in an enlarged thyroid (goiter).
- Importantly, TSH does not regulate the peripheral conversion of T4 to T3, a process primarily occurring in the liver and kidneys.
- The actions of TSH on the thyroid gland can be divided into three time-dependent effects:
- Hormonal Interactions:
- In addition to regulating thyroid function, TRH also influences the secretion of other pituitary hormones, including growth hormone (GH), follicle-stimulating hormone (FSH), and prolactin (PRL).
- Noradrenaline stimulates TRH secretion, while somatostatin and serotonin inhibit it. These interactions highlight the intricate hormonal balance maintained by the HPT axis.
Vasculature of Thyroid Gland
The thyroid gland’s operation depends on its vasculature as it guarantees a sufficient blood flow for metabolic activities and hormone production. The thyroid’s great vascularization helps hormones be quickly delivered into the circulation. Anatomical relevance of the thyroid gland’s venous drainage and artery supply is discussed in this part.
- Arterial Supply- Two primary arteries Supply the thyroid gland most of their blood:
- Superior Thyroid Artery
- Originating as the first branch of the external carotid artery, this artery is
- Supplying the top portion of the thyroid gland, it runs downhill and forward.
- Clinically, especially during surgical operations, its closeness to the external branch of the superior laryngeal nerve, which innervates the larynx, is significant.
- Inferior Thyroid Artery:
- Rising from the thyrocervical trunk—a branch of the subclavian artery—this artery serves the bottom half of the thyroid gland.
- Its anatomical link is important during thyroid operations as it runs directly next to the recurrent laryngeal nerve, which also innervates the larynx.
- Thyroid Ima Arterial:
- About 10% of the population carries this extra artery, which comes out of the brachiocephalic trunk.
- It provides the thyroid gland’s front surface and isthmus, therefore offering a different blood supply path—which might be rather important in some therapeutic situations.
- Superior Thyroid Artery
- Venous Drainage– The thyroid gland’s venous drainage is achieved by the following veins:
- Superior Thyroid Vein: This vein flows into the internal jugular vein from the top section of the thyroid gland.
- Middle Thyroid Vein: Also emptying into the internal jugular vein, it gathers blood from the gland’s lateral sides.
- Inferior Thyroid Vein: This vein discharges into the brachiocephalic vein after draining the lowest parts of the thyroid. Surrounded by a venous plexus, the thyroid gland allows effective blood outflow and preserves venous return to the systemic circulation.
Functions of Thyroid Gland
Maintaining metabolic equilibrium and facilitating human body growth and development depend on the thyroid gland performing several important roles. Its main function is related to the synthesis of hormones that greatly affect several physiological mechanisms.
- Generation of Thyroid Hormones– Two main hormones produced by the thyroid gland are triiodothyronine (T3) and thyroxine (T4), both of which are very vital for metabolic activity. These hormones contain iodine, which is essential for their production, therefore stressing the part the gland plays in iodine metabolism.
- Control of Basal Metabolic Rate – The basal metabolic rate—that is, the rate the body uses during rest—is fundamentally regulated by thyroid hormones. They affect several metabolic routes, including glucose, lipid, and protein metabolism, thereby fostering energy generation and use.
- Effects on Development and Enhancement– Particularly in areas like the brain and kidneys, optimal development and growth depend on thyroid hormones. Development of brain tissues and skeletal growth in children depends on appropriate amounts of these hormones.
- Calcium Level Control – Made by the parafollicular cells of the thyroid gland, calcitonin helps control blood calcium ion levels. This hormone lowers blood calcium levels by suppressing osteoclastic activity in bones, therefore limiting the calcium output into circulation.
- Affect on Iodine Metabolism– The thyroid gland guarantees that the body’s iodine levels are kept sufficient as it is the principal organ in charge of iodine metabolism; this is essential for the creation of thyroid hormones.
- Support of Cellular Metabolism– Almost all tissues rely on thyroid hormones to boost oxygen intake and generate heat generation, therefore stimulating metabolic activities. Both thermoregulation and general energy balance depend on this metabolic stimulus.
- Feedback systems and homeostasis– Feedback systems involving the pituitary gland control thyroid hormone production, especially with regard to thyroid-stimulating hormone (TSH). This control guarantees that hormone levels stay within a certain range to satisfy physiological needs of the organism.
- Bursuk, E. (2012). Introduction to Thyroid: Anatomy and Functions. InTech. doi: 10.5772/37942
- Allen E, Fingeret A. Anatomy, Head and Neck, Thyroid. [Updated 2023 Jul 24]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470452/
- Khan YS, Farhana A. Histology, Thyroid Gland. [Updated 2022 Dec 5]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK551659/
- https://www.onlinebiologynotes.com/what-is-thyroid-gland/
- https://www.gastroepato.it/en_tiroide.htm
- https://teachmephysiology.com/endocrine-system/thyroid-parathyroid-gland/thyroid-gland/
- https://reference.medscape.com/article/835535-overview?form=fpf
- https://teachmeanatomy.info/neck/viscera/thyroid-gland/
- https://en.wikipedia.org/wiki/Thyroid
- https://oer.unimed.edu.ng/LECTURE%20NOTES/1/2/Dr-Akpalaba-Immaculata-ANATOMY-OF-THE-THYROID-GLAND.pdf
- https://www.medicalnewstoday.com/articles/thyroid-gland-function
- https://www.thoughtco.com/thyroid-gland-anatomy-373251
- https://www.geeksforgeeks.org/thyroid-gland-anatomy-function-and-clinical-aspects/
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.