What is Root Culture?
- Root culture is a specific technique in plant tissue culture that focuses on the cultivation of excised radical tips from aseptically germinated seeds. This process occurs in a liquid nutrient medium, where the roots are induced to grow independently under carefully controlled environmental conditions. By isolating and cultivating root systems, researchers can investigate various aspects of root development, physiology, and response to environmental stimuli.
- The initial phase of root culture involves the germination of seeds in sterile conditions to ensure that no contaminants are present. Once the seeds have germinated, the radical tips—often referred to as root tips—are excised. This surgical removal must be executed with precision to preserve the viability of the root tissue. The excised tips are then placed into a nutrient-rich liquid medium designed to support their growth and development.
- In the liquid medium, root tips are provided with essential nutrients, including carbohydrates, minerals, and growth regulators. These components facilitate the physiological processes necessary for root growth, such as cell division, elongation, and differentiation. As the roots grow, they can be monitored for various parameters, including growth rate, morphological changes, and the influence of external factors like light and temperature.
- Moreover, root culture is particularly valuable for studying root architecture and function. Researchers can examine how roots respond to different nutrient concentrations, hormonal signals, and environmental stressors. This detailed analysis is crucial for understanding the mechanisms underlying nutrient uptake, water absorption, and overall plant health.
- Additionally, root culture holds significant potential in agricultural and horticultural applications. By optimizing conditions within the culture system, scientists can develop rootstocks with enhanced resistance to diseases or environmental stresses. This application is particularly important in the context of sustainable agriculture, where resilient root systems can improve crop yields and reduce the need for chemical inputs.
Principle of Root Culture
- The principle of root culture revolves around the cultivation of excised root tips from aseptically germinated seeds in a controlled environment. This technique is essential for studying root development, as intact plants often present challenges for isolating root tips due to their deep burial in soil. Additionally, young root tips from seedlings are highly sensitive to toxic sterilants, making surface sterilization impractical. Therefore, the focus shifts to utilizing excised radicle tips, which can be more effectively cultured under sterile conditions.
- Initiating root culture involves the careful excision of root tips from germinated seeds, which must be done under aseptic conditions to prevent contamination. These excised root tips are then placed in a moving liquid nutrient medium designed to support root growth and development. This medium typically contains essential nutrients, growth regulators, and vitamins that mimic the natural soil environment, facilitating optimal conditions for root growth.
- In culture, the excised root tips are induced to grow similarly to the root system of an intact plant. This process allows researchers to observe and measure root growth in a controlled setting. Various parameters can be evaluated, including fresh and dry weight, increase in the length of the main root axis, the number of emergent lateral roots, and the total length of lateral roots per culture. These measurements provide insights into root development and overall plant health.
- Moreover, root cultures can be sustained through a technique known as subculturing. This involves repeatedly cutting and transferring the main root tips or lateral tips into fresh nutrient medium at regular intervals. This practice not only prolongs the life of the culture but also allows for the establishment of clones from a single root culture. Consequently, root culture can serve as an effective means of propagating plants with desirable traits, enhancing research in plant breeding and genetics.
- Therefore, the principle of root culture emphasizes the importance of excised root tips in studying root growth and development under sterile conditions. By providing optimal growth conditions and employing techniques such as subculturing, researchers can explore various aspects of root physiology and ecology. This methodology contributes significantly to the understanding of plant root systems, their responses to environmental stimuli, and their role in overall plant health.
Protocol of Root Culture
Below is a detailed protocol outlining the processes involved in initiating root cultures and clones.
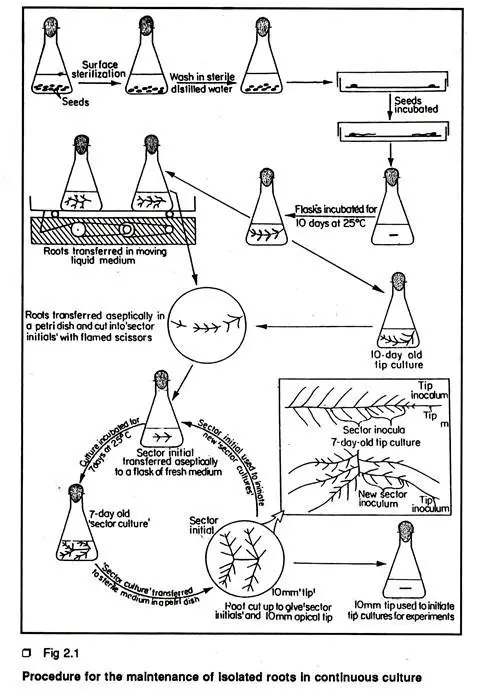
- Initiation of Isolated Root Culture:
- Surface Sterilization and Germination:
- Seeds are subjected to conventional surface sterilization methods.
- Post-sterilization, the seeds are germinated on moist filter paper or White’s basal medium at a temperature of 25°C in a dark environment.
- Excising Root Tips:
- When the seedling roots reach a length of 20 to 40 mm, excise 10 mm apical tips (referred to as tip inoculum) using a scalpel.
- Transfer each excised tip to 40 ml of liquid medium contained within 100 ml wide-necked Erlenmeyer flasks.
- Incubation:
- Incubate the flasks at 25°C in the dark to promote root growth.
- Surface Sterilization and Germination:
- Initiation of Clones:
- The root material obtained from a single radicle tip can be multiplied and maintained in continuous culture. These genetically uniform root cultures are termed clones of isolated roots. The initiation of root clones is a straightforward process.
- Protocol for Initiating Root Clones:
- Establish a Root Culture:
- Begin by establishing a root culture from the radical tip of a seed, following the previously outlined procedure.
- Transferring to Petri Dish:
- After establishing the root culture, transfer a 10-day-old root culture to a sterile petri dish containing sterile medium.
- Using flamed scissors, cut the main axis of the root into several pieces, known as sector inoculum or initial, with each piece containing four or five young lateral roots.
- Inoculation to Liquid Medium:
- Aseptically transfer each individual sector inoculum to a flask containing liquid medium and incubate in the dark at 25°C.
- Further Culture Initiation:
- The sector culture can be utilized to initiate further tip cultures using 10 mm apical tips of the laterals from a growing sector inoculum.
- Alternatively, the growing sector can be divided into 4-5 sectors to initiate additional sector cultures.
- Establish a Root Culture:
Importance of Root Culture
Root culture is a critical area of plant biotechnology that contributes significantly to our understanding of root physiology and various applications in plant science. It facilitates the study of root development and function, revealing insights that can be applied in agriculture, medicine, and environmental science. The following points summarize the importance of root culture based on existing research and findings.
- Basic Information and Knowledge Advancement:
- Carbohydrate Metabolism and Mineral Nutrition:
- Root cultures have enhanced the understanding of carbohydrate metabolism in roots and the roles of mineral ions and vitamins in promoting root growth.
- Dependence on Shoot Hormones:
- Research has indicated that roots rely on shoots for the supply of growth hormones, thereby highlighting the interconnectedness of plant organs.
- Root Clone Studies:
- Genetically uniform root clones provide an ideal platform for investigating the effects of various compounds on root growth and development, enabling more controlled experiments.
- Carbohydrate Metabolism and Mineral Nutrition:
- Specific Applications of Root Culture:
- Study of Nodulation in Leguminous Roots:
- The formation of nodules on leguminous roots by nitrogen-fixing bacteria (such as Rhizobium sp.) is a complex physiological process. Root cultures of leguminous plants offer an optimal system for studying this phenomenon.
- A modified technique was developed by M. Raggio, N. Raggio, and J. G. Torrey, wherein the base of an excised root of Phaseolus vulgaris is supplied with sucrose and vitamins via agar medium, while the rest of the root is in contact with an inorganic nitrate-free medium containing Rhizobium.
- This method allows isolated roots to develop nodules, thereby facilitating in vitro studies on the relationship between symbiotic nitrogen-fixing bacteria and host plants.
- Regeneration of Shoots from Roots:
- Isolated root cultures can be maintained for extended periods; however, in some species such as Atropa and Convolvulus arvensis, it is possible to induce shoot regeneration from cultured roots.
- Shoot primordia may originate from callus formation at the cut ends of the roots or from internal root tissues, providing insights into regenerative processes that hold both practical and theoretical significance.
- Synthesis of Secondary Metabolites:
- Roots of many medicinal plants produce important alkaloids and other secondary metabolites as by-products of their metabolic processes. Root culture techniques have been instrumental in locating the sites of synthesis for these compounds.
- Additionally, nutritional manipulations in root cultures can enhance the production of these valuable metabolites, supporting pharmaceutical applications.
- Initiation and Development of Secondary Vascular Tissues:
- Typically, excised cultured roots exhibit only primary structures; however, research has shown that excised root tips from Pisum sativum can develop vascular cambium when cultured in a medium supplemented with indoleacetic acid (IAA).
- Torrey utilized a modified technique to study this phenomenon, where the basal portions of excised roots were exposed to various growth substances. The results indicated that additional sucrose and auxins play a critical role in cambial development, suggesting potential pathways for enhancing secondary vascular tissue formation.
- Study of Nodulation in Leguminous Roots: