Describe the molecular structure of phospholipids with reference to their hydrophilic (polar) phosphate heads and hydrophobic (non-polar) fatty acid tails
Describe the molecular structure of phospholipids with reference to their hydrophilic (polar) phosphate heads and hydrophobic (non-polar) fatty acid tails
Please login to submit an answer.
Phospholipids are a unique class of lipids that form the primary structure of cell membranes. Their molecular structure consists of both hydrophilic (polar) and hydrophobic (non-polar) regions, making them amphipathic molecules. This amphipathic nature allows them to form bilayers in aqueous environments, which is essential for cellular compartmentalization and membrane function.
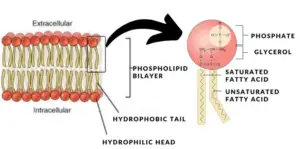
Molecular Structure of Phospholipids
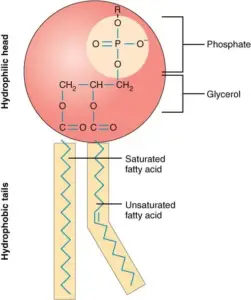
- Glycerol Backbone:
- Phospholipids have a glycerol molecule as their backbone. This three-carbon molecule serves as the central framework to which other groups are attached.
- Hydrophilic (Polar) Phosphate Head:
- Attached to one of the glycerol’s hydroxyl groups is a phosphate group (PO₄³⁻), which is polar and negatively charged.
- The phosphate group is often further modified by an additional polar group, such as choline, serine, or ethanolamine, creating a hydrophilic “head”.
- This hydrophilic head is attracted to water molecules, making it soluble in aqueous environments.
- Hydrophobic (Non-Polar) Fatty Acid Tails:
- The remaining two hydroxyl groups of glycerol are each bonded to a fatty acid chain through ester bonds.
- These fatty acid tails are non-polar and hydrophobic, meaning they repel water. The fatty acids can be either:
- Saturated (with no double bonds, creating straight chains) or
- Unsaturated (with one or more double bonds, causing kinks in the chains).
- The hydrophobic tails are insoluble in water, which drives them to cluster together away from aqueous environments.
Arrangement in Cell Membranes
In aqueous environments, phospholipids naturally arrange themselves into a bilayer, with the hydrophilic heads facing outward toward the water on either side of the membrane, and the hydrophobic tails facing inward, away from water. This arrangement is fundamental to the formation of cell membranes:
- Selective Barrier: The hydrophobic interior of the bilayer acts as a barrier to most water-soluble substances, helping regulate what enters and exits the cell.
- Membrane Fluidity: The unsaturated fatty acid tails in some phospholipids introduce kinks, preventing tight packing and allowing the membrane to remain fluid and flexible.
- Structural Integrity: The hydrophilic and hydrophobic regions maintain the structural integrity of the membrane, providing stability while also allowing dynamic movement of molecules within the bilayer.
Amphipathic Nature of Phospholipids
The structure of phospholipids gives them an amphipathic nature, meaning they have both hydrophilic and hydrophobic parts. This unique characteristic allows them to arrange themselves in specific ways in water:
- Bilayer Formation: In aqueous environments, phospholipids naturally align to form a bilayer, with the hydrophilic phosphate heads facing outward toward the water on either side and the hydrophobic fatty acid tails facing inward, away from the water. This arrangement forms the fundamental structure of cell membranes.
- Barrier and Selectivity: The bilayer acts as a selective barrier, allowing non-polar, small molecules to pass through while preventing large, polar molecules and ions from freely crossing, thus maintaining a controlled internal environment for the cell.

- Share on Facebook
- Share on Twitter
- Share on LinkedIn
Helpful: 0%




