What is Plant Transformation?
- Plant transformation is a critical biotechnological process aimed at introducing foreign genes into the genomes of plants. This technique enhances various agronomic traits, including increased yield, improved quality, and enhanced tolerance to biotic and abiotic stresses. Traditional genetic improvement methods have yielded significant advancements in crop species, but modern plant engineering techniques have expanded the toolkit available to plant breeders, thus enhancing breeding efficiency.
- The process of plant transformation encompasses several methodologies, each with distinct mechanisms and applications. Electroporation is one such technique, which employs electrical pulses to facilitate the introduction of DNA into plant cells. Biolistic bombardment, also known as microprojectile bombardment, utilizes high-velocity microprojectiles to deliver genetic material directly into plant cells. Protoplast fusion is another method that involves the merging of plant cells without cell walls, allowing for genetic material exchange. Additionally, the Agrobacterium tumefaciens-mediated gene transfer technique exploits the natural ability of this soil bacterium to transfer DNA into plant cells, thus enabling genetic modification.
- Each of these transformation techniques necessitates careful preparation and manipulation of plant cells prior to genomic alterations. The initial steps involve isolating and preparing plant cells, followed by the transformation process, which includes the introduction of the foreign DNA. Subsequently, identification and selection of transformants are performed to ensure the successful integration of the desired genes. Finally, the transformed cells must be regenerated into whole plants, which requires specific conditions and expertise.
- It is important to note that these traditional transformation methods often demand a high level of technical skill and specialized equipment, making them resource-intensive. In contrast, the pollen tube-mediated gene transfer (PTT) method offers a more straightforward alternative. This technique simplifies the process by avoiding the conventional regeneration protocols associated with other transformation systems. The PTT method utilizes the natural growth of pollen tubes to deliver DNA directly into the ovule, thereby streamlining the transformation process.
What is Pollen Tube-Mediated Gene Transfer?
- Pollen tube-mediated gene transfer (PTT) is a pioneering method that facilitates the introduction of exogenous DNA into the plant embryo, initially demonstrated in cotton (Gossypium hirsutum) in 1983. This innovative approach harnesses the natural process of pollen tube growth, allowing for a more efficient and direct method of genetic transformation.
- The PTT method begins with the careful removal of the stigma from the recipient plant shortly after pollination. This step is crucial as it prepares the flower for the subsequent application of a solution containing the desired exogenous DNA. The DNA solution is applied to the severed style of the plant, enabling the foreign genetic material to be transported through the elongating pollen tube as it grows toward the ovary.
- Once the pollen tube reaches the ovary, the exogenous DNA is incorporated into the fertilized zygote of the recipient plant. This integration occurs at a critical stage of embryo formation, ensuring that the foreign genes become part of the recipient’s genome. As a result, transformed seeds are produced that carry the new genetic information.
- A significant advantage of the PTT method is that it does not require protoplast manipulation, cell culture, or the extensive regeneration procedures commonly associated with other transformation techniques. This characteristic simplifies the overall process, making it more accessible and less resource-intensive compared to methods such as Agrobacterium-mediated transformation or biolistic bombardment.
- Moreover, the PTT approach addresses some of the limitations often encountered in traditional plant transformation techniques. For instance, it mitigates issues related to fertility reduction, genotype specificity, and genetic variation, including mutations and altered transgene expression that may arise from stress-induced responses during tissue culture. Therefore, the PTT method not only accelerates the gene transfer process but also enhances the reliability of genetic transformation in plants.
Requirement for Pollen Tube-Mediated Gene Transfer
The pollen tube pathway method requires several key components and conditions to be effective in transforming cotton plants. Here are the main requirements:
- Flowering Cotton Plants: The method necessitates the use of healthy, flowering cotton plants that are at the appropriate stage of development, typically when the flowers are fertilized and the pollen tube is formed.
- Transgenic DNA Solution: A solution containing the desired transgenic DNA is essential. This DNA usually includes:
- A gene of interest that confers a specific trait (e.g., pest resistance).
- A promoter that regulates the expression of the gene.
- Optionally, a marker gene that allows for the selection of successfully transformed plants.
- Injection Equipment: A fine needle or micro-injection apparatus is required to inject the DNA solution directly into the ovary of the flower. Precision is crucial to ensure that the DNA is delivered effectively.
- Sterile Conditions: Maintaining sterile conditions during the injection process is important to prevent contamination that could affect the success of the transformation.
- Growth Conditions: After injection, the ovules must be allowed to develop into seeds under appropriate growth conditions, including adequate light, temperature, and moisture.
- Screening Techniques: Following seed maturation, molecular techniques such as PCR (Polymerase Chain Reaction) or Southern blot analysis are needed to screen the resulting plants for successful integration of the transgenic DNA.
- Regulatory Compliance: Depending on the region, compliance with local regulations regarding the use of genetically modified organisms (GMOs) may be necessary, including obtaining permits and conducting environmental assessments.
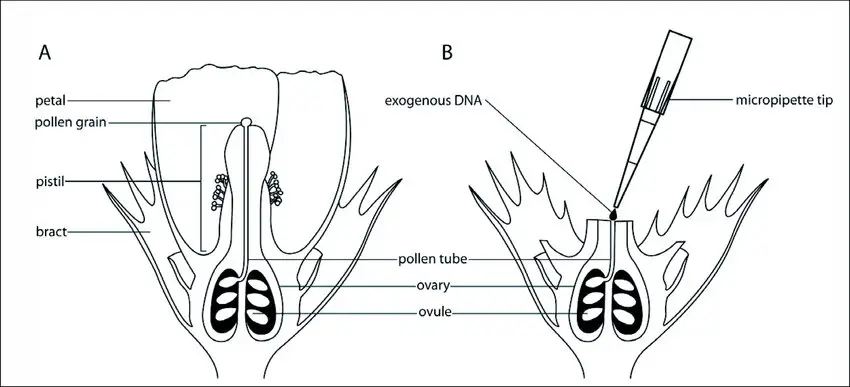
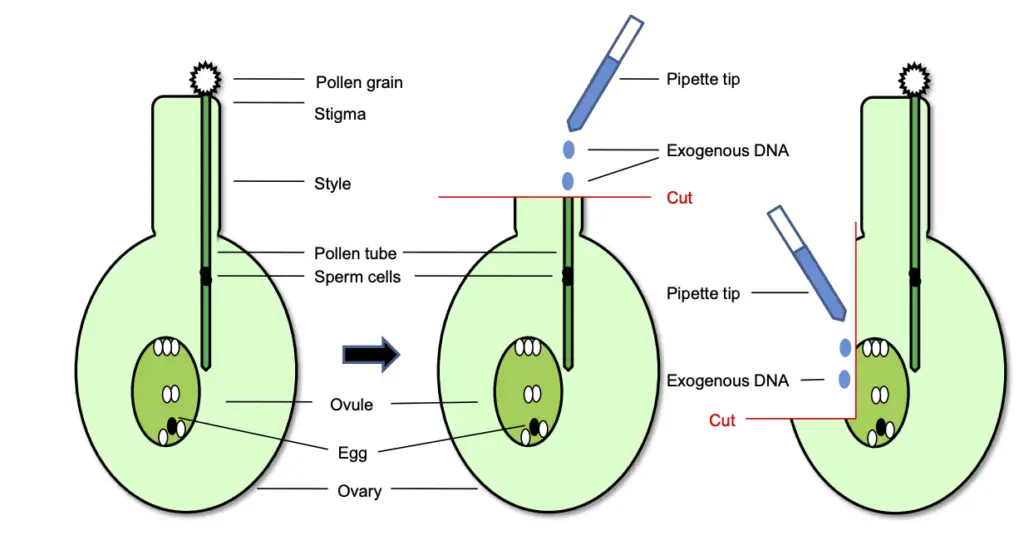
Steps for Pollen Tube-Mediated Gene Transfer
Pollen tube-mediated gene transfer (PTT) is a cutting-edge technique that facilitates genetic transformation in plants by leveraging the natural process of fertilization. This method involves several key steps, each crucial for ensuring the successful introduction of exogenous DNA into the plant genome. Below is a detailed breakdown of the steps involved in PTT:
- Pollen Grain Preparation: The process begins with the collection of pollen grains, which consist of a vegetative cell and a generative cell. The pollen grains adhere to the stigma of the recipient plant, initiating the germination process.
- Pollen Tube Formation: Once the pollen grain is in place, the vegetative cell develops into a pollen tube. This tube extends through the style, eventually penetrating the ovule. Concurrently, the generative cell divides to produce two sperm cells, which are essential for fertilization.
- Mechanical Cutting of the Style: Shortly after pollination, the style of the recipient plant is mechanically cut. This step is critical as it allows for the application of exogenous DNA directly onto the severed style. The removal of the stigma facilitates easier access for DNA transfer.
- Application of Exogenous DNA: An exogenous DNA solution is applied to the cut style of the recipient plant. This DNA is intended to be transported via the growth of the pollen tube into the ovule.
- Delivery to the Ovule: As the pollen tube grows, it carries the exogenous DNA toward the ovary. The goal is for the DNA to reach the ovule, where it can integrate with the undivided but fertilized zygote.
- Integration into the Genome: Once the DNA reaches the ovule, it is incorporated into the genome of the recipient plant during the embryo formation stage. This successful integration ensures that the foreign genes are present in the resulting transformed seeds.
- Alternative Methods for DNA Delivery: Besides the mechanical cutting of the style, an additional technique involves directly delivering the exogenous DNA into the ovule through an “ovary drip” method. This approach entails cutting and opening the ovary to facilitate the introduction of the DNA, ensuring that it effectively reaches the ovule.
Advantages
The pollen tube pathway method offers several advantages for the transformation of cotton plants and other flowering plants. Here are the key benefits:
- No Need for Tissue Culture: Unlike other transformation methods such as Agrobacterium-mediated transformation or particle bombardment, the pollen tube pathway does not require the use of cell or tissue cultures. This makes it applicable to a wider variety of cotton cultivars, including those that are difficult to regenerate from tissue cultures.
- Direct Transformation: The method allows for the direct injection of DNA into the ovary of the flower, utilizing the natural fertilization process. This can lead to more efficient integration of the transgene into the plant’s genome.
- Broader Applicability: Since it does not rely on specific cultivars that can regenerate from tissue cultures, the pollen tube pathway can be used with any cotton cultivar, increasing the flexibility of the transformation process.
- Reduced Resource Requirements: The absence of tissue culture means that fewer resources (time, materials, and labor) are needed for the transformation process, making it more cost-effective.
- Higher Success Rates: The method can potentially yield higher success rates in terms of transgene integration and expression, as it directly targets the ovule during a critical stage of development.
- Simplified Selection Process: The pollen tube pathway does not require a marker gene for selection, as other methods (e.g., PCR, Southern blot analysis) can efficiently determine whether a plant is transgenic. This can simplify the selection process and reduce the complexity of the transformation.
- Potential for Enhanced Traits: The method allows for the introduction of novel traits that may not be achievable through conventional breeding, such as pest resistance or improved agronomic characteristics, thereby enhancing crop performance.
Limitations
While the pollen tube pathway method has several advantages, it also comes with certain limitations. Here are the key drawbacks associated with this transformation technique:
- Inconsistent Results: The pollen tube pathway method can sometimes yield inconsistent or irreproducible results. Variability in the transformation efficiency may occur due to differences in the developmental stage of the flowers or environmental conditions.
- Limited to Flowering Plants: This method is specifically designed for flowering plants, which limits its applicability to non-flowering species. This restricts the range of plants that can be transformed using this technique.
- Technical Skill Required: The injection process requires a high level of precision and technical skill. Improper injection can lead to damage to the flower or ovary, reducing the chances of successful transformation.
- Potential for Low Transformation Rates: Although the method can be efficient, the actual transformation rates may still be lower compared to other methods like Agrobacterium-mediated transformation, which has been shown to have higher success rates in some studies.
- Regulatory Challenges: As with all methods of creating genetically modified organisms (GMOs), the pollen tube pathway method may face regulatory hurdles. Compliance with local and international regulations regarding GMOs can complicate research and commercialization efforts.
- Dependency on Pollen Tube Development: The success of the method relies on the proper development of the pollen tube and the timing of the injection. If the pollen tube is not adequately formed or if the timing is off, the chances of successful DNA integration decrease.
- Limited Knowledge Base: Compared to more established methods like Agrobacterium-mediated transformation, there is less extensive research and knowledge available regarding the pollen tube pathway method. This can make troubleshooting and optimization more challenging.
- Potential for Genetic Instability: As with other transformation methods, there is a risk of genetic instability in the resulting transgenic plants, which can affect the expression of the transgene in subsequent generations
Applications
The pollen tube pathway method has several applications in plant biotechnology, particularly in the development of transgenic crops. Here are some key applications:
- Genetic Modification for Pest Resistance: The method is used to introduce genes that confer resistance to pests, such as the incorporation of Bacillus thuringiensis (Bt) genes into cotton plants. This enhances the plants’ ability to withstand pest attacks, reducing the need for chemical pesticides.
- Improvement of Agronomic Traits: Researchers utilize the pollen tube pathway to introduce genes that improve various agronomic traits, such as drought tolerance, disease resistance, and enhanced nutritional content. This can lead to better crop yields and sustainability in agriculture.
- Development of Novel Varieties: The technique allows for the creation of new cotton varieties with specific traits that may not be achievable through traditional breeding methods. This includes traits like fiber quality improvement and enhanced growth rates.
- Research in Plant Development: The pollen tube pathway method can be employed in research to study gene function and expression in flowering plants. By introducing specific genes, scientists can investigate their roles in plant development and physiology.
- Biotechnology in Developing Countries: The method is particularly useful in developing countries where traditional breeding methods may be limited. It provides a means to rapidly develop improved crop varieties that can help address food security and agricultural challenges.
- Marker-Free Transformation: Since the pollen tube pathway does not necessarily require a marker gene for selection, it can be used to create transgenic plants that do not carry additional selectable marker genes. This is beneficial for regulatory approval and consumer acceptance of genetically modified crops.
- Hybrid Seed Production: The method can be applied in the production of hybrid seeds by introducing desirable traits into specific parental lines, thereby enhancing the performance of hybrid varieties in the market.
- Exploration of Gene Functionality: The technique allows for the exploration of gene functionality in a more natural context, as it utilizes the plant’s reproductive system for transformation, which can lead to more stable expression of the introduced traits.
- Wang XF, Tao YB, Lu YT. Pollen tubes enter neighbouring ovules by way of receptacle tissue, resulting in increased fruit-set in Sagittaria potamogetifolia Merr. Ann Bot. 2002 Jun;89(6):791-6. doi: 10.1093/aob/mcf136. PMID: 12102535; PMCID: PMC4233842.
- Lora J, Hormaza JI, Herrero M. The Diversity of the Pollen Tube Pathway in Plants: Toward an Increasing Control by the Sporophyte. Front Plant Sci. 2016 Feb 9;7:107. doi: 10.3389/fpls.2016.00107. PMID: 26904071; PMCID: PMC4746263.
- Showalter, Ann & Heuberger, Shannon & Tabashnik, Bruce & Carriere, Yves & Coates, Brad. (2009). A Primer for Using Transgenic Insecticidal Cotton in Developing Countries. Journal of insect science (Online). 9. 22. 10.1673/031.009.2201.
- Ali, Asjad & Bang, Sun & Chung, Sang-Min & Staub, Jack. (2014). Plant Transformation via Pollen Tube-Mediated Gene Transfer. Plant Molecular Biology Reporter. 33. 10.1007/s11105-014-0839-5.
- Lamport, Derek & Tan, Li & Held, Michael & Kieliszewski, Marcia. (2017). Pollen tube growth and guidance: Occam’s razor sharpened on a molecular arabinogalactan glycoprotein Rosetta Stone. New Phytologist. 217. 10.1111/nph.14845.