What is Plasmodium vivax?
- Plasmodium vivax is a protozoan parasite that plays a significant role as a human pathogen, primarily responsible for recurring malaria infections. This parasite is the most prevalent species among those that cause malaria, and while it is generally less virulent than Plasmodium falciparum—the deadliest malaria species—it can still lead to severe complications, including splenomegaly, which is an abnormal enlargement of the spleen. The disease associated with P. vivax is particularly noteworthy for its potential to cause serious illness and death, emphasizing the need for awareness and control measures.
- The transmission of Plasmodium vivax occurs through the bite of infected female Anopheles mosquitoes, as the males do not feed on blood. The geographical distribution of P. vivax is extensive, with significant populations found in Asia, Latin America, and select regions in Africa. Initially believed to have originated in Asia, recent studies have revealed that closely related parasites are endemic to wild chimpanzees and gorillas in central Africa, suggesting an African origin for human P. vivax. Notably, P. vivax accounts for approximately 65% of malaria cases in Asia and South America.
- One of the distinguishing characteristics of P. vivax is its ability to undergo sporogonic development in the mosquito host at lower temperatures, a feature not shared with P. falciparum. This adaptability increases the likelihood of transmission in temperate climates, where at least 71 mosquito species can act as vectors for the parasite. It is estimated that around 2.5 billion people globally are at risk of contracting P. vivax malaria, with the burden of risk varying by region.
- In terms of regional contributions to the global risk of P. vivax infections, the Americas represent about 22% of the area at risk; however, due to sparsely populated high-endemic areas, the population at risk is only around 6%. Africa, on the other hand, has a limited transmission of P. vivax, primarily because many individuals lack the Duffy antigen, which the parasite requires for entry into red blood cells. As a result, stable transmission is confined mostly to Madagascar and parts of the Horn of Africa, representing about 3.5% of the global population at risk.
- Central Asia has the highest percentage of the global population at risk, accounting for 82% of cases, with significant populations in India and Myanmar. Southeast Asia has notable endemic areas, particularly in Indonesia and Papua New Guinea, contributing approximately 9% to the global population at risk.
- Malaria remains a critical public health concern worldwide, as evidenced by the approximately 229 million malaria cases and around 409,000 deaths reported in 2019, predominantly in Africa. The Plasmodium genus comprises five species known to infect humans, including P. vivax, which, despite causing fewer fatalities than P. falciparum, contributes significantly to global morbidity and mortality associated with malaria.
The life cycle of Plasmodium vivax
The life cycle of Plasmodium vivax is divided into two phage;
- Asexual cycle or Life Cycle of Plasmodium vivax in Man or Schizogony in man
- Sexual Cycle or Life cycle Plasmodium vivax in mosquito or Sexual Cycle in mosquito
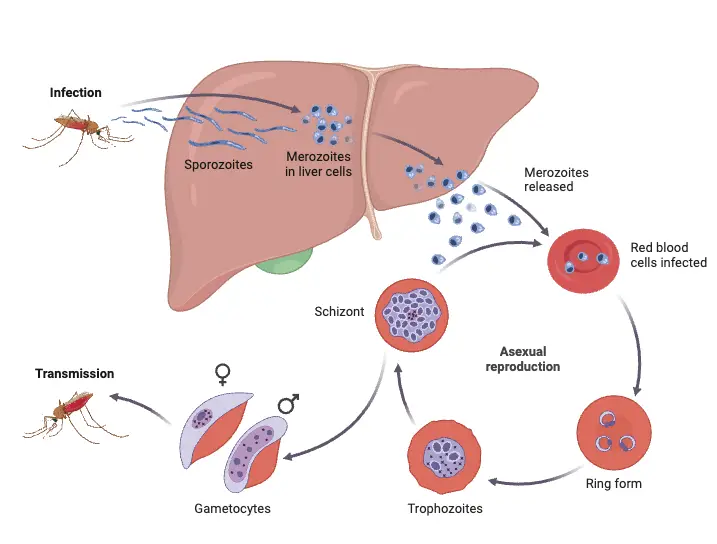
1. Asexual cycle or Life Cycle of Plasmodium vivax in Man or Schizogony in man
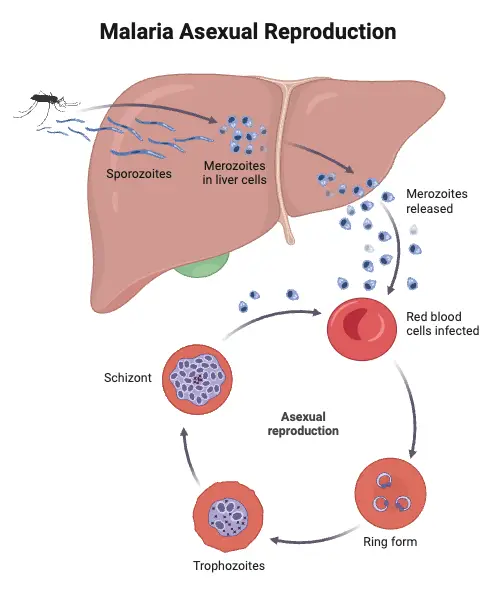
Below is a detailed explanation of the asexual cycle of Plasmodium vivax in Man;
- Infection Process
- The cycle begins when an infected female Anopheles mosquito bites a healthy person to feed on blood.
- During this process, the mosquito introduces sporozoites, the infective form of Plasmodium vivax, into the bloodstream through its saliva.
- Sporozoites quickly disperse into the host’s system, leading to subsequent stages of infection.
- Sporozoites
- Sporozoites are the initial infective forms of the parasite, measuring approximately 11 to 12 µm in length and 0.5-1 µm in width.
- They are spindle-shaped and characterized by a single, vesicular nucleus containing a nucleolus.
- Covered by an elastic pellicle, sporozoites possess longitudinal microtubules that enable wriggling movements, facilitating their entry into host cells.
- The anterior end features an apical cap, which contains secretory organelles that assist in penetrating liver cells.
- Liver Schizogony
- Within approximately 30 minutes after infection, sporozoites exit the bloodstream and enter liver parenchymal cells, where they undergo asexual reproduction through schizogony.
- Liver schizogony comprises two distinct phases: pre-erythrocytic and exo-erythrocytic.
- Pre-erythrocytic Phase
- Inside liver cells, sporozoites grow and transform into larger, spherical forms known as schizonts.
- Schizonts undergo multiple fission, producing thousands of merozoites.
- Upon rupture of the schizonts, merozoites are released into the liver’s sinusoids as cryptozoites, which are resistant to medications and host immune responses.
- This phase lasts about 8-10 days, during which the blood remains free of parasites, and no symptoms are exhibited.
- Exo-erythrocytic Phase
- In this phase, cryptozoites enter new liver cells and continue to multiply, forming a reservoir of merozoites.
- The process can yield two types of metacryptozoites: micro metacryptozoites (small and numerous) and macro metacryptozoites (larger and less numerous).
- Micro metacryptozoites enter RBCs to initiate the erythrocytic phase, while macro metacryptozoites reinvade fresh liver cells, perpetuating the exo-erythrocytic phase.
- The parasites in this stage evade the host’s immune defenses and remain unaffected by antimalarial drugs.
- Pre-patent and Incubation Periods
- The time from the initial infection by sporozoites until the first detection of parasites in the blood is termed the pre-patent period, lasting about 8 days for P. vivax.
- The interval between infection and the onset of the first malarial symptoms constitutes the incubation period, averaging 10-17 days.
- Erythrocytic Schizogony
- The third phase, erythrocytic schizogony, occurs when micro metacryptozoites invade RBCs.
- Each metacryptozoite enters a single RBC, progressing through several stages: trophozoite, signet ring, amoeboid, and finally, schizont.
- In the trophozoite stage, the parasite enlarges by consuming hemoglobin from the RBCs, while the signet ring stage is marked by a large vacuole that pushes the nucleus to the cell’s periphery.
- The trophozoite further develops into an amoeboid form, which then multiplies asexually within the schizont, producing 12 to 24 merozoites.
- The rupture of the erythrocyte releases merozoites back into the bloodstream, where they can infect new RBCs, continuing the cycle approximately every 48 hours.
- Post-erythrocytic Schizogony
- Occasionally, some merozoites produced in the erythrocytic phase will reinvade liver cells, undergoing another cycle of schizogony known as pre-erythrocytic schizogony.
- Formation of Gametocytes
- After numerous cycles of asexual reproduction, some merozoites differentiate into gametocytes, the sexual forms of the parasite.
- Gametocytes are of two types: macrogametocytes (female) and microgametocytes (male).
- Macrogametocytes are larger, dark, and contain more food reserves.
- Microgametocytes are smaller, motile, and have less cytoplasmic reserves, appearing lighter in color.
- Gametocytes do not undergo division while in the human bloodstream; they must be ingested by an Anopheles mosquito to complete their development. If not, they will degenerate or die.
2. Sexual Cycle or Life cycle Plasmodium vivax in mosquito or Sexual Cycle in mosquito
Below is a detailed explanation of the sexual cycle of Plasmodium vivax in mosquito;
- Ingestion by Mosquito
- Female Anopheles mosquitoes feed on the blood of infected humans, which contains gametocytes, the sexual form of Plasmodium vivax, along with other erythrocytic stages such as merozoites.
- Upon ingestion, most components, including red blood cells (RBCs), are digested in the mosquito’s stomach; however, the gametocytes survive the digestive process, allowing the lifecycle to progress.
- Gametogenesis/Gametogony (Formation of Gametes)
- The formation of gametes from gametocytes occurs through a process known as gametogenesis or gametogony.
- Two types of gametes are produced: microgametes (male) and macrogametes (female).
- Microgametogenesis:
- Microgametocytes undergo ex-flagellation in the mosquito’s midgut, stimulated by the cooler body temperature of the mosquito.
- During this process, the nucleus of the microgametocyte divides meiotically to form 6-8 daughter nuclei. These nuclei then migrate toward the periphery, along with the cytoplasm, forming flagella-like structures.
- Consequently, 6-8 motile microgametes, approximately 20-25 microns in length, emerge and actively seek out macrogametes in the mosquito’s stomach.
- Macrogametogenesis:
- Macrogametocytes mature, pushing out two polar bodies to develop into female gametes, also known as macrogametes or megagametes.
- The macrogamete is non-motile and develops a specialized structure called the cone of reception, which aids in fertilization.
- Fertilization
- Upon encountering a microgamete, the male gamete enters the female gamete at the site of the fertilization cone.
- This fusion results in the formation of a diploid zygote, or synkaryon, through a process known as syngamy. It is classified as anisogamous, as the uniting gametes exhibit distinct morphological differences.
- Zygote formation occurs approximately 9 to 10 days post-blood meal.
- Ookinete Formation
- The zygote initially remains rounded and motionless for about 24 hours, subsequently elongating into a motile, worm-like structure known as an ookinete.
- Measuring approximately 15-22 microns in length and 3 microns in width, ookinetes penetrate the midgut wall of the mosquito, facilitated by lytic secretions.
- Electron microscopy reveals a central irregular nucleus, dense cytoplasm, brown pigment granules, and numerous mitochondria, indicating robust protein synthesis during this stage.
- Encystment
- After penetrating the midgut wall, ookinetes settle beneath the thin membrane separating the midgut from the hemocoel.
- Here, they undergo a transformation into a spherical shape and form a cystic structure known as an oocyst.
- The oocyst derives its cyst wall partially from ookinete secretions and partially from the mosquito’s stomach tissue. It expands in size, and multiple oocysts, numbering up to 500, can be observed adhering to the stomach wall.
- Sporogony
- Each oocyst enters a phase of asexual reproduction known as sporogony, which involves the production of sporozoites from the zygote nucleus via a process of multiple fission.
- The oocyst undergoes division, first meiotically and then mitotically, leading to the formation of numerous haploid nuclei within sporoblasts over a period of 2-3 days.
- These nuclei further multiply, and cytoplasm constricts around them, leading to the elongation of sporozoites, which exhibit a slender or sickle shape.
- An individual oocyst can produce up to 10,000 sporozoites, which gather around vacuoles within the oocyst.
- The process of sporogony is completed within 10 to 20 days, depending on environmental temperature.
- Once mature, the oocyst ruptures, releasing thousands of motile sporozoites into the mosquito’s hemocoel.
- Transmission to Humans
- The released sporozoites travel to the salivary glands of the mosquito, entering the duct of the hypopharynx.
- During subsequent blood meals, these sporozoites are injected into the bloodstream of a new host, thereby initiating the next cycle of infection and perpetuating the life cycle of Plasmodium vivax.
Reference
- Menkin-Smith L, Winders WT. Plasmodium vivax Malaria. [Updated 2023 Jul 17]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK538333/
- https://www.vivaxmalaria.org/p-vivax-malaria-an-introduction/lifecycle-of-plasmodium-vivax-malaria
- https://www.geeksforgeeks.org/plasmodium-life-cycle/
- https://microbiologynotes.com/life-cycle-of-plasmodium-vivax/
- https://www.researchgate.net/figure/Plasmodium-vivax-lifecycle-in-human-host-P-vivax-is-transmitted-to-humans-by-the-bite_fig1_292189586
- https://www.notesonzoology.com/protozoa/life-cycle-of-plasmodium-vivax-with-diagram-protozoa/5694
- https://www.drishtiias.com/daily-news-analysis/p-ovale-malaria
- https://www.cdc.gov/dpdx/malaria/index.html
- https://en.wikipedia.org/wiki/Plasmodium_vivax