What are Helminths?
- Helminthes, commonly referred to as worms, represent a diverse group of multicellular invertebrates characterized by elongated bodies, either flat or round. This group is primarily divided into two major classes: the roundworms, scientifically known as Nematoda, and the flatworms, classified as Platyhelminthes. Notably, among the many species within these categories, approximately half exhibit parasitic behavior, with some recognized as significant pathogens affecting human health.
- The structure and developmental stages of helminths are critical to understanding their biology and the diseases they cause. Helminths undergo a life cycle that includes egg, larval (or juvenile), and adult stages. These various life stages are crucial in the study of epidemiology and the pathogenesis of helminthic infections. Detailed knowledge of these stages facilitates effective diagnosis and treatment of individuals infected by these parasites.
- In terms of anatomy, helminths possess organ systems similar to other animals, yet their parasitic adaptations often result in reduced complexity. For instance, many parasitic helminths exhibit diminished digestive and nervous systems, as well as limited locomotor capabilities, reflecting their dependency on host organisms for survival. The outermost layer of helminths is typically a protective covering known as the cuticle or tegument, which plays a vital role in nutrient absorption and protection against the host’s immune response.
- Flatworms, or platyhelminths, include notable subcategories such as flukes and tapeworms. The term “platy” derives from the Greek word for “flat,” indicative of their morphology. Conversely, roundworms, or nematodes, are named from the Greek root meaning “thread,” highlighting their elongated shape. Both groups can be further categorized based on their residing organs within hosts; for example, lung flukes, extraintestinal tapeworms, and intestinal roundworms. This classification aids in the understanding of their ecological roles and interactions with hosts.
- The reproductive strategies of helminths are equally diverse. Some species are monoecious, containing both male and female reproductive organs within a single individual, while others are dioecious, featuring distinct male and female organisms. In general, most adult helminths produce eggs that are expelled through the host’s excretions, contributing to the lifecycle’s continuity and potential spread of infections.
- Additionally, the anatomical features of helminths reflect their functional adaptations. For instance, flukes and cestodes have specialized structures such as suckers (acetabula) or false suckers (bothria) that assist in adhering to host tissues. Male nematodes possess unique external modifications of the cuticle that serve as accessory sex organs. Notably, tapeworms lack an alimentary canal entirely, absorbing nutrients directly through their tegument, showcasing a remarkable evolutionary adaptation to their parasitic lifestyle.
Classification of Helminths
Helminths, a diverse group of invertebrates, are primarily recognized for their elongated, flat, or round bodies. They are categorized into two main classes: flatworms (Platyhelminthes), which include flukes (trematodes) and tapeworms (cestodes), and roundworms (Nematoda). The classification of helminths is essential for understanding their biology, life cycles, and modes of infection.
- Flatworms (Platyhelminthes):
- Trematodes (Flukes):
- Morphology: Flukes are typically leaf-shaped and range in size from a few millimeters to 8 centimeters.
- Reproductive Characteristics: Most flukes are hermaphroditic, possessing both male and female reproductive organs, which allows for self-fertilization and cross-fertilization.
- Life Cycle: Trematodes undergo multiple larval stages. In humans, adult flukes reside in the lumen of blood vessels, particularly in the case of blood flukes (schistosomes), which are unique as they are the only bisexual flukes affecting humans.
- Transmission: Eggs are expelled in the feces, urine, or sputum, and upon reaching aquatic environments, they hatch into ciliated larvae. These larvae either penetrate or are consumed by snail intermediate hosts, eventually leading to the development of cercariae, which migrate to the definitive host.
- Common Trematodes:
- Lung Fluke: Paragonimus westermani
- Intestinal Flukes: Fasciolopsis buski, Heterophyes heterophyes, Mettgonimus yokagawi
- Liver Flukes: Clonorchis sinensis, Opisthorchis spp., Fasciola hepatica
- Blood Flukes: Schistosoma mansoni, Schistosoma haematobium, Schistosoma japonicum
- Cestodes (Tapeworms):
- Morphology: Tapeworms are elongated, segmented flatworms that inhabit the gastrointestinal tract of humans and other animals.
- Reproductive Characteristics: They are also hermaphroditic, with segments called proglottids that vary in length from 2 millimeters to 10 meters. Adult tapeworms can have from several to thousands of segments.
- Life Cycle: Eggs are released when gravid proglottids are shed into the intestine, subsequently passed in the stool. The eggs develop into different larval stages within both immediate hosts (crustaceans) and intermediate hosts (vertebrates) before reaching maturity in the definitive human host.
- Common Cestodes:
- Taenia saginata, Taenia solium, Diphyllobothrium latum, Hymenolepis nana, Echinococcus spp.
- Trematodes (Flukes):
- Roundworms (Nematodes):
- Morphology: Nematodes are cylindrical in shape and are primarily bisexual, with fertilization occurring through copulation.
- Reproductive Characteristics: Most parasitic nematodes lay eggs that, when expelled from the host, contain either a zygote or a fully formed larva.
- Life Cycle: The developmental process includes egg, larval, and adult stages. Some nematodes, such as filariae and Trichinella spiralis, deposit larvae directly into host tissues.
- Transmission Modes:
- Direct Infection: Occurs when eggs are transmitted directly from anus to mouth without requiring an environmental reservoir (e.g., Enterobius vermicularis [pinworm/threadworm] and Trichuris trichiura [whipworm]).
- Modified Direct Infection: Involves eggs that must incubate in soil before becoming infectious (e.g., Ascaris lumbricoides [roundworm]).
- Skin Penetration: Used by hookworms such as Ancylostoma duodenale and Necator americanus, which enter the host through the skin.
Characteristics of Helminths
- Size and Structure:
- Helminths are generally large organisms, with a minimum size of over 1 mm and some species reaching lengths greater than 1 m.
- The body structure of helminths varies: flatworms, such as flukes and tapeworms, exhibit a dorsoventrally flattened morphology, while roundworms, or nematodes, possess a cylindrical shape.
- Body Coverings:
- Flatworms have a body covered by a plasma membrane, which serves to protect the organism and facilitate nutrient absorption.
- In contrast, roundworms are enveloped in a cuticle that provides structural support and protection against the host’s immune responses.
- Organ Systems:
- Helminths possess well-developed organ systems, including a complete alimentary canal, excretory systems, and reproductive organs.
- The alimentary canal enables these parasites to actively feed on host tissues or fluids, thus securing the nutrients necessary for their growth and reproduction.
- Reproductive Strategies:
- Many helminths are hermaphroditic, containing both male and female reproductive organs within a single individual. This adaptation enhances reproductive success by allowing self-fertilization or cross-fertilization.
- Others exhibit separate sexes, which may lead to more complex reproductive interactions. For example, in certain species, males and females engage in close physical association during mating, allowing for more effective reproduction.
- Feeding Mechanisms:
- Helminths are generally active feeders, utilizing specialized mouthparts and muscular structures to ingest their food. This is particularly evident in roundworms, where the esophagus serves as a muscular pump to draw food into the intestine.
- In contrast, some flatworms absorb nutrients directly through their tegument, allowing them to derive sustenance from the surrounding host environment.
- Lifecycle Complexity:
- Helminths often undergo complex life cycles involving multiple developmental stages and hosts. For instance, many flukes and tapeworms require intermediate hosts, such as snails or fish, to complete their life cycles before infecting a definitive host, often a mammal or human.
- This complexity enhances their ability to survive in various ecosystems and adapt to changing environmental conditions.
- Adaptive Features:
- The presence of specialized structures, such as suckers or hooks in flatworms, enables these parasites to firmly attach to host tissues, ensuring a stable environment for feeding and reproduction.
- Nematodes possess features like a bursa, a flap-like structure that aids in copulation, highlighting the evolutionary adaptations that facilitate successful mating.
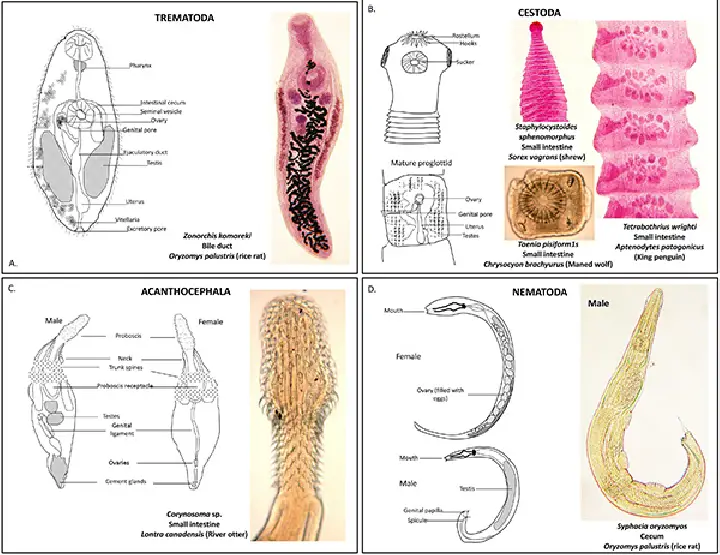
Structure of Helminths
The structure of helminths encompasses a diverse array of organisms, primarily classified into three main groups: flukes (trematodes), tapeworms (cestodes), and roundworms (nematodes). Each of these groups exhibits distinct morphological features and physiological adaptations that contribute to their survival and reproductive success. Understanding these structural differences is essential for comprehending their life cycles, modes of infection, and mechanisms of interaction with their hosts.
- Flukes (Trematodes)
- Flukes exhibit a dorsoventrally flattened body with bilateral symmetry, a characteristic of platyhelminths. Their leaf-shaped form can range from a few millimeters to approximately 7-8 cm in length.
- The tegument is a complex structure that serves multiple functions, including protection and absorption.
- Flukes possess an oral sucker surrounding the mouth and a ventral sucker or acetabulum, which allows them to attach to host tissues. Notably, they lack a true body cavity, with their organs embedded in specialized connective tissue known as parenchyma.
- The muscular layers, composed of somatic muscle, extend through the parenchyma and are attached to the tegument, facilitating movement and stability.
- Their alimentary canal includes a muscular pharynx, esophagus, and a branched intestine lined with a single layer of epithelial cells. The intestine may end blindly or open into an excretory vesicle.
- The excretory system is of the protonephridial type, featuring flame cells that function to excrete waste products. Flame cells direct tissue filtrate through canals into the main collecting ducts.
- Flukes are predominantly hermaphroditic, with both male and female reproductive organs present in a single individual. Male reproductive structures typically consist of two testes and accessory glands, while females possess a single ovary, a seminal receptacle, and yolk glands, with eggs produced in the ootype.
- The reproductive process includes both self-fertilization and cross-fertilization. Eggs, usually operculated, exit through the genital pore, contributing to the life cycle of the flukes that often involves aquatic environments and snail hosts.
- Tapeworms (Cestodes)
- Unlike flukes, tapeworms are elongated and segmented, consisting of numerous repeating units called proglottids. Their lengths can range from 2-3 mm to several meters.
- The tapeworm body is divided into three main regions: the scolex (head), the neck (region of segment proliferation), and the strobila (chain of proglottids). The scolex, which contains attachment organs such as bothria, suckers, or a rostellum, facilitates adherence to the intestinal walls of the host.
- Adult tapeworms notably lack an alimentary canal, absorbing nutrients through the tegument, which is adapted with microvilli to enhance absorption.
- Tapeworms are also hermaphroditic, with each proglottid containing male and female reproductive systems. Egg deposition mechanisms differ between groups; for example, eggs of pseudophyllidean tapeworms exit through a uterine pore, while cyclophyllidean eggs are released when gravid proglottids are shed.
- The eggs of tapeworms can be operculated or non-operculated, depending on the species, and contain embryos that develop into various larval forms in intermediate hosts.
- Roundworms (Nematodes)
- Nematodes, or roundworms, are characterized by their cylindrical shape, in contrast to the flattened bodies of platyhelminths. Their outer body wall consists of a chemically complex, noncellular cuticle, a thin hypodermis, and a layer of somatic musculature.
- The body cavity is a pseudocoelom, lacking a mesothelium lining, and contains fluid along with celomocytes, which are fixed cells associated with the longitudinal cords.
- The alimentary canal is complete, featuring both a mouth and an anus. The mouth is surrounded by lips with sensory papillae, and the muscular esophagus plays a crucial role in food ingestion.
- The roundworm excretory system varies, with some possessing a gland and pore system while others have a more complex tubular excretory system. This system is vital for waste removal and osmoregulation.
- Nematodes typically exhibit sexual dimorphism, with males being smaller and possessing distinctive copulatory structures such as spicules. Their reproductive systems include tubular structures for both sexes, leading to the external environment through the vulva.
- The developmental process in nematodes encompasses distinct stages: eggs, multiple larval stages, and the adult form. The larvae undergo several molts, shedding the cuticle at each stage until reaching maturity.
Life cycle of Helminths
The life cycle of helminths, specifically flukes, tapeworms, and roundworms, is a complex process involving various developmental stages and often multiple hosts. Understanding these life cycles is crucial for grasping how these parasites infect and survive within their definitive hosts, including humans.
- Flukes (Trematodes):
- Flukes exhibit a dorsoventrally flattened body and possess bilateral symmetry, with a distinct anterior end. Their leaf-shaped bodies can range from a few millimeters to 7-8 cm in length.
- Flukes possess a complex tegument, an oral sucker surrounding the mouth, and a ventral sucker (acetabulum) for attachment to host tissues. They lack a true body cavity, with organs embedded in specialized connective tissue called parenchyma.
- The alimentary canal is well developed, featuring a muscular pharynx and branched intestines, which may have secondary and tertiary branches. The excretory system, comprised of flame cells, serves to eliminate waste.
- Trematodes are primarily hermaphroditic, containing both male and female reproductive organs. The male typically has two testes and a cirrus, while the female has a single ovary and vitellaria (yolk glands). Fertilization may occur through self-fertilization or cross-fertilization, with eggs passing through the genital pore.
- Flukes undergo a generalized life cycle involving multiple larval stages, requiring at least two hosts, one of which is always a snail. The cycle begins with eggs being excreted in feces, urine, or sputum, which then hatch into ciliated larvae (miracidia). These larvae penetrate or are ingested by a snail.
- Inside the snail, the miracidium develops into a sporocyst, which may produce rediae or daughter sporocysts. Cercariae emerge from the snail and can penetrate the definitive host directly, develop encysted metacercariae in a second intermediate host, or encyst on aquatic vegetation. Ingesting a metacercarial cyst results in the maturation of the fluke within a specific organ of the definitive host.
- Tapeworms (Cestodes):
- Tapeworms exhibit a flattened, elongated structure divided into segments called proglottids. They vary in size from 2-3 mm to up to 10 m and lack an alimentary canal, absorbing nutrients directly through their tegument.
- The anatomy includes a scolex (head) for attachment, a neck for segment proliferation, and a strobila (chain of proglottids). The reproductive system is hermaphroditic, with each proglottid containing both male and female organs.
- Tapeworms shed eggs differently than flukes; the eggs can exit through a uterine pore or through gravid proglottids, which disintegrate to release eggs in feces.
- The life cycle of tapeworms also involves multiple hosts. The eggs develop into an oncosphere, which can transform into various larval forms, such as cysticerci or plerocercoids, depending on the species and intermediate hosts involved. For example, the oncosphere of pseudophyllidean tapeworms develops into a coracidium, while cyclophyllidean tapeworms develop into cysticerci in specific hosts.
- Roundworms (Nematodes):
- Nematodes, or roundworms, are cylindrical and possess a body wall made up of a complex outer cuticle, a thin hypodermis, and a layer of somatic musculature. The body structure includes a pseudocoelom, which aids in the movement and transport of nutrients.
- Their alimentary canal is complete, featuring a mouth, muscular esophagus, and anus. The mouth is equipped with sensory papillae, and the intestines are lined with microvilli.
- Nematodes are usually bisexual, with males being smaller and possessing specialized copulatory structures. The female reproductive system includes reflexed ovaries leading to a tubular uterus.
- Fertilization typically requires copulation, though some species exhibit parthenogenesis. The developmental stages of nematodes include eggs, larvae, and adults. Each larval stage undergoes a molt, resulting in four larval stages followed by the adult form.
- Nematodes can produce eggs containing either an undeveloped zygote or a larva, with some, such as filariae, depositing larvae directly into host tissues. The lifecycle often involves multiple larval molts, leading to the eventual maturation into adults capable of reproduction.
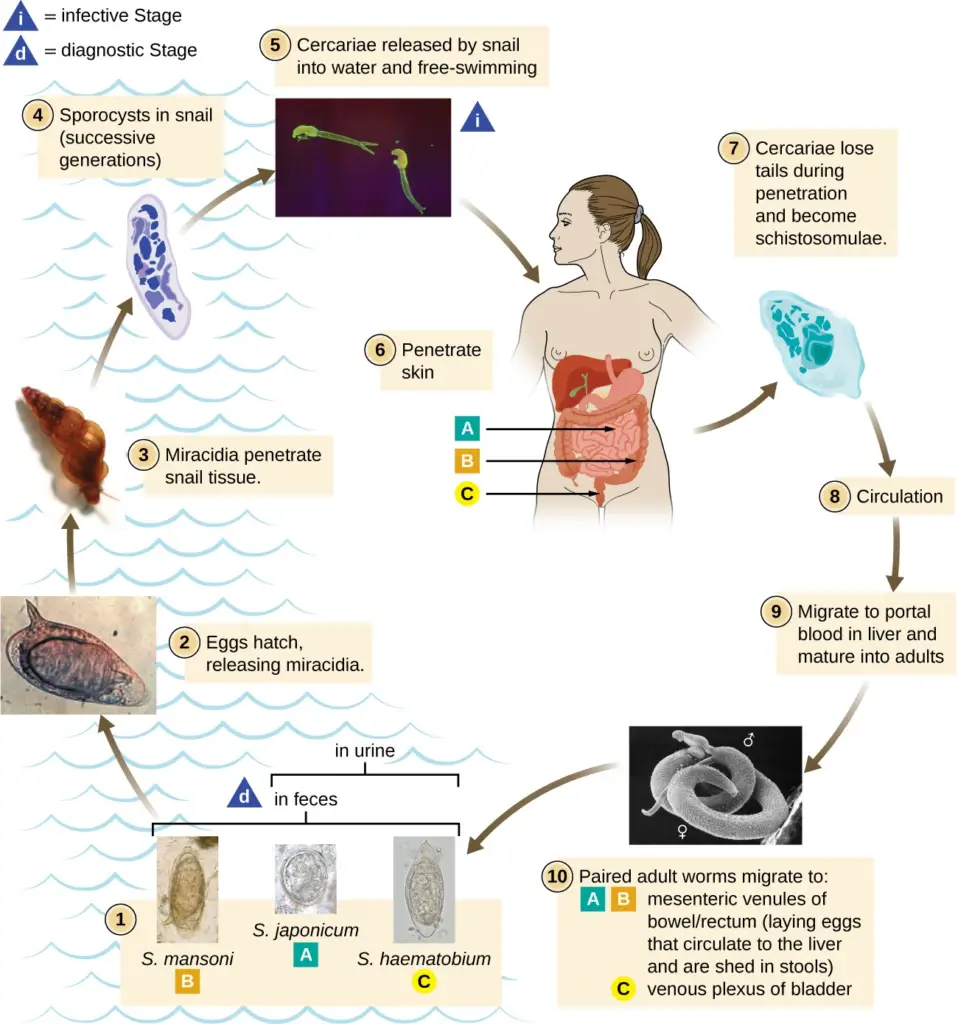
Infection of Helminths
The infection process of helminths involves a complex interplay between the parasites and their hosts, characterized by various transmission routes and susceptibility factors. Understanding this process is critical for preventing and managing helminth infections, which pose significant health risks in many populations.
- Transmission Methods:
- Helminths can be transmitted to humans through multiple pathways. One of the most straightforward methods is the accidental ingestion of infective eggs or larvae. Common examples include Ascaris, Echinococcus, Enterobius, and Trichuris.
- Some helminths utilize a more invasive approach; larvae may actively penetrate the skin. Notable examples include hookworms and schistosomes, which enter the host through direct contact with contaminated environments.
- Additionally, certain helminths rely on intermediate hosts for transmission. In these cases, an arthropod vector may bite the host to take a blood meal, thereby transmitting infective stages, as observed in filarial worms. Other helminths, like Clonorchis, require humans to consume infected intermediate hosts, such as fish or meat, to become infected.
- Environmental Influences:
- The prevalence of helminth infections in human populations is influenced by various environmental factors. Hygiene practices play a critical role, as many helminth eggs and larvae are excreted in urine and feces.
- Climate conditions may also favor the survival and transmission of infective stages. For example, warm, moist environments are conducive to the lifecycle of many helminths, enhancing their potential to infect humans.
- Food preparation practices can further influence infection risk. Inadequate cooking or improper handling of contaminated food can lead to increased transmission of helminths.
- Host Factors Influencing Susceptibility:
- Human behavior significantly impacts susceptibility to helminth infection. Individuals who do not maintain proper hygiene are more likely to encounter infective stages, resulting in higher rates of infection.
- Importantly, helminths do not replicate within a single host, meaning the level of infection correlates directly with the number of infective stages a person encounters. Nevertheless, not every exposure leads to an established infection, as many infective organisms are eliminated by the host’s nonspecific defense mechanisms.
- Various factors affect a host’s immune response and susceptibility. For instance, children often exhibit greater susceptibility to helminth infections, frequently displaying higher infection rates than adults. Similarly, the immune response may diminish with age, contributing to increased infection rates in older individuals.
- Genetic variability among individuals can also influence susceptibility, with some being predisposed to heavier infections. Nutritional status and immune responses during pregnancy or lactation further modulate susceptibility, as does concurrent infection with other pathogens or the use of immunosuppressive therapies.
- Parasite Adaptations:
- Helminths possess several adaptations that enhance their ability to evade host defenses. Their size and mobility often render many host defense mechanisms ineffective. Notably, some species, such as Ascaris and tapeworms, can grow several centimeters to over a meter in length, complicating host attempts to eliminate them.
- The tough cuticle that covers adult roundworms provides further protection against host immune responses. Additionally, their ability to navigate through host tissues allows them to evade localized inflammatory responses, thereby promoting their survival.
- The long-term presence of these parasites can lead to chronic inflammation, tissue damage, and immune stimulation, ultimately impacting the host’s health. The physical burden of large parasites can also contribute to significant morbidity.
Pathogenesis of Helminths
The pathogenesis of helminths involves a multifaceted interaction between the parasites and their hosts, leading to a range of direct and indirect pathological effects. Understanding these mechanisms is crucial for comprehending the health impacts of helminth infections, which can result in significant morbidity.
- Direct Damage from Worm Activity:
- Helminths can cause significant physical damage to internal organs, primarily through blockage and pressure effects. For example, large helminths like Ascaris and certain tapeworms may obstruct the intestines, potentially leading to life-threatening complications, particularly after treatment interventions. Migrating Ascaris can also block bile ducts, resulting in cholestasis.
- Granulomas often form around schistosome eggs in the liver, impeding blood flow and causing liver pathology. Additionally, adult Wuchereria present in lymphatics can obstruct lymph flow, resulting in conditions like elephantiasis.
- Larval forms, such as those from Echinococcus granulosus, may create large, fluid-filled cysts in various organs, including the liver, brain, and lungs, leading to pressure atrophy. Cysts formed by Taenia solium in the central nervous system can provoke neurological symptoms due to the pressure exerted on surrounding tissues.
- The presence of intestinal worms induces various pathological changes in the intestinal mucosa, with hookworms actively sucking blood from mucosal capillaries. Their anticoagulant secretions prolong bleeding, leading to significant blood loss, anemia, and protein deficiency, particularly in malnourished individuals. Inflammatory responses to other intestinal worms can cause protein-losing enteropathies.
- While worms compete for host nutrients, their direct interference with digestion and absorption processes is more concerning, exacerbating nutritional deficiencies. For example, the tapeworm Diphyllobothrium latum can lead to vitamin B12 deficiency due to its absorption of this crucial nutrient.
- Migration and Tissue Damage:
- Many helminths migrate extensively through host tissues, resulting in both direct tissue damage and the initiation of hypersensitivity reactions. Commonly affected organs include the skin, lungs, liver, and intestines. Migration can lead to clinical manifestations such as petechial hemorrhages, pneumonitis, eosinophilia, urticaria, pruritus, organomegaly, and granulomatous lesions.
- Feeding behavior of helminths on host tissues contributes to pathology, particularly through the induction of hyperplastic and metaplastic changes in epithelial tissues. For instance, liver fluke infections cause hyperplasia of bile duct epithelium. Chronic inflammation surrounding parasites, like the granulomas formed around schistosome eggs in the bladder wall, is linked with the development of neoplasia, although the exact nature of this relationship remains unclear.
- The continuous release of excretory-secretory materials by living worms can directly affect host cells and tissues, contributing to overall pathology.
- Indirect Damage from Host Response:
- The immune response to helminths plays a significant role in the pathogenesis of infections. All helminths are perceived as foreign bodies by the host immune system, leading to immunological reactions. The interplay between direct damage caused by parasites and the indirect damage due to host immune responses complicates the pathology.
- In schistosome infections, particularly those caused by Schistosoma mansoni, hypersensitivity reactions to eggs trapped in the liver can cause blood flow obstruction, leading to significant liver damage. Similarly, filarial infections can lead to lymphatic obstruction, compounding the impact of the parasites.
- Immune-mediated inflammatory responses occur in various tissues, including the skin, lungs, liver, intestines, central nervous system, and eyes, as worms migrate through these structures. Systemic effects such as eosinophilia, edema, and joint pain reflect local allergic responses to the parasites.
- In intestinal infections, particularly with Strongyloides and Trichinella, structural changes such as villous atrophy and increased mucosal permeability occur. These alterations can lead to fluid accumulation in the gut lumen and reduced intestinal transit time, potentially resulting in protein-losing enteropathy. The inflammatory response from schistosome egg passage through the intestinal wall can also induce severe intestinal pathology, with heavy whipworm infections leading to blood loss and rectal prolapse.
- Chronic Infections and Long-Term Consequences:
- The chronic nature of helminth infections contributes to the severity of indirect pathological changes. Many helminths have long lifespans, leading to irreversible inflammatory changes that produce functional impairments in tissues.
- Examples of long-term effects include hyperplasia of bile ducts associated with chronic liver fluke infections, extensive fibrosis linked to chronic schistosomiasis, and skin atrophy resulting from onchocerciasis. Severe pathology may also arise when helminths migrate to abnormal body sites, exacerbating the host’s condition.
Defenses Against Helminths Infection
Defenses against helminth infections involve a complex interplay between host immune responses and the parasitic strategies employed by the worms to evade these defenses. Understanding these mechanisms is essential for developing effective interventions and treatments for helminth infections.
- Nonspecific Resistance:
- Upon attempting to enter the host, helminths face a range of nonspecific defenses that are designed to inhibit the invasion of pathogens. When parasites are ingested orally, they must endure the harsh acidic environment of the stomach before reaching the small intestine. While natural human parasites have adapted to withstand these conditions, opportunistic parasites often do not survive.
- Additionally, the environmental conditions in the small bowel can be hostile for accidental parasites, which may lack the adaptations necessary for survival and development. If helminths penetrate the intestinal wall, they can trigger inflammatory responses, which serve to immobilize and kill the invading worms. However, this inflammation can also lead to significant pathology, as seen in infections caused by Anisakis.
- Helminths entering the body through the skin must navigate the skin’s protective secretions, penetrate the epidermal layers, and evade inflammatory reactions in the dermis. For example, larvae of dog and cat hookworms (Ancylostoma spp.) can induce dermatitis, creating visible trails in the skin due to inflammatory responses.
- Once helminths invade tissues, they require specific environmental signals to mature properly. A lack of these signals can inhibit development, although it does not necessarily lead to the death of the parasite. For instance, in cases of Toxocara infection, prolonged larval survival can result in ongoing inflammatory responses that cause tissue damage.
- Specific Acquired Immunity:
- Specific immunity plays a critical role in host defense against helminths, although distinguishing between nonspecific and specific immune mechanisms can be challenging. All helminths elicit robust immune responses, which can be detected through measurements of specific antibodies or cellular immunity.
- Despite the presence of strong immune responses, protective immunity against helminths in humans appears to be weak or lacking. This observation is supported by the high prevalence of infections in endemic regions and the tendency for individuals to remain infected for prolonged periods, even after treatment.
- Nonetheless, some degree of immunity is evident, as the intensity of infections often diminishes with age, and many individuals in endemic areas remain free of clinical symptoms. Research indicates that antibodies targeting helminth surface antigens may enhance complement- or cell-mediated damage to the worms. The primary effector cells involved in this immune response are macrophages and eosinophils, with immunoglobulins IgM, IgG, and IgE playing significant roles.
- Antibodies may also inhibit the activity of enzymes released by worms, interfering with their ability to penetrate host tissues or feed. During acute inflammation, structural and physiological changes in the intestine can help dislodge intestinal worms. Evidence suggests that IgE-mediated hypersensitivity reactions, which involve mast cells and basophils, may contribute to this process, although concrete evidence is still lacking.
- Interestingly, while IgA is abundant in the intestinal lumen, its role in providing protective immunity against helminths in humans remains inconclusive. Some studies indicate potential protective effects, but further research is needed.
- Avoidance of Host Defenses:
- Despite the host’s immune responses, many helminths manage to survive within their hosts for extended periods. Various factors contribute to their resilience, including their size and motility, but they also employ sophisticated mechanisms to evade host defenses.
- Some helminths, such as schistosomes, can mask their outer surfaces by acquiring host molecules, thereby reducing their antigenicity. Filarial nematodes can cover their cuticles with serum albumin, providing a further disguise against immune detection.
- Additionally, many helminths secrete substances that can depress lymphocyte function, inactivate macrophages, or digest antibodies. Larval cestodes may release anticomplement factors that protect their surfaces from immune lysis. Although true antigenic variation is not commonly observed, some helminth species exhibit stage-specific changes in antigens during development, which can delay effective immune responses.
- Helminths release substantial amounts of antigenic material, which may divert or exhaust the host’s immune responses. This antigenic overload can lead to the production of non-protective antibodies that may block the action of potentially protective ones, as seen in schistosomiasis.
- Immune Suppression:
- Many helminth infections are associated with a degree of immune suppression, affecting both specific and general immune responsiveness. Potential explanations for this immune suppression include antigen overload, competition among antigens, the induction of suppressor cells, and the production of factors that specifically inhibit lymphocyte activity.
- Reduced immune responsiveness can prolong the survival of existing helminths and may increase the host’s susceptibility to other pathogens. Epidemiological evidence suggests that early-life infections, particularly those acquired shortly after birth, may induce a form of immune tolerance that allows substantial helminth burdens to persist in the host.
Diagnosis and treatment of helminth infections
The diagnosis and treatment of these infections necessitate a thorough understanding of the specific parasites involved, their life cycles, and the respective therapeutic interventions.
- Enterobiasis (Enterobius vermicularis): This is the most common helminthic infection in Western Europe. Adult female pinworms deposit eggs in the perianal folds, leading to auto-infection through scratching and subsequent ingestion. Transmission occurs through contaminated surfaces or food. While most cases are asymptomatic, pruritus ani is prevalent, and higher worm burdens can result in abdominal pain and nausea.
- Diagnosis typically involves the pinworm paddle or tape test, which entails applying tape to the perianal area at night for microscopic examination.
- Treatment involves administering mebendazole, 100 mg as an immediate dose. In the event of re-infection, the same dose may be repeated after two weeks for affected individuals aged six months and older. Mebendazole is contraindicated in pregnancy and breastfeeding. Patients should be advised to maintain strict hygiene practices to prevent reinfection and ensure that bedding and clothing are adequately cleaned.
- Ascaris (Ascaris lumbricoides): Commonly known as the common roundworm, Ascaris infections can lead to serious complications such as intestinal obstruction and respiratory symptoms due to larval migration, known as Ascaris pneumonitis.
- Diagnosis can be confirmed through stool microscopy, where larvae and eosinophils may be identified in sputum, particularly during A. pneumonitis.
- For treatment, adults over 18 years typically receive albendazole (400 mg) or mebendazole (500 mg) as an immediate dose. Pediatric dosing varies: children aged 1–2 years receive mebendazole 100 mg twice daily for three days, while children aged 2–18 years receive 100 mg twice daily or a single 500 mg dose.
- Toxocara spp. (Toxocara canis and Toxocara catis): These roundworms primarily infect dogs and cats, with human infection occurring through ingestion of eggs from contaminated environments.
- Infections may result in visceral larva migrans (VLM), presenting with symptoms like pneumonitis and hepatosplenomegaly. Ocular larva migrans (OLM) may also occur, leading to visual disturbances.
- Diagnosis involves serological testing, with eosinophilia being indicative of infection.
- Treatment for confirmed VLM includes albendazole (400 mg twice daily for seven days) in adults, while ocular cases require urgent ophthalmological evaluation.
- Taenia spp. (Taenia saginata and Taenia solium): These tapeworms can cause taeniasis and cysticercosis, respectively. Symptoms often include gastrointestinal disturbances, and T. solium may lead to more severe complications like epilepsy due to cysts in the brain.
- Diagnosis involves stool microscopy to confirm the presence of proglottids or eggs.
- For T. saginata, adults receive niclosamide (2 g) or praziquantel (10 mg/kg). In contrast, T. solium infections, particularly with neurological involvement, necessitate careful management of seizures with anticonvulsants alongside potential antihelminthic treatment.
- Fasciola hepatica: This fluke infection is primarily associated with sheep-rearing areas. Symptoms may range from mild to severe, including right upper quadrant pain and hepatomegaly.
- Diagnosis typically involves stool microscopy for eggs, and serological tests can confirm the presence of the parasite.
- Treatment for Fascioliasis is triclabendazole (10 mg/kg daily for two days), although it is contraindicated in pregnant and breastfeeding women.
- Schistosomiasis: Caused by blood flukes like Schistosoma mansoni, this infection often presents with minimal symptoms initially but can progress to severe chronic complications affecting the liver and intestines.
- Diagnosis is confirmed through stool or urine microscopy for eggs and serological tests.
- Praziquantel (20 mg/kg) is the standard treatment, typically administered in two doses.
- Hookworm infections (Ancylostoma duodenale and Necator americanus): Predominantly asymptomatic, hookworm infections can lead to anemia and gastrointestinal symptoms.
- Diagnosis involves stool microscopy, often identifying eosinophilia.
- Treatment includes albendazole (400 mg) or mebendazole (100 mg twice daily for three days).
- Whipworm (Trichuris trichiura): Although often asymptomatic, heavy infestations can cause significant gastrointestinal distress and anemia.
- Diagnosis typically involves stool microscopy.
- Treatment options include albendazole (400 mg) or mebendazole (500 mg).
- Echinococcus spp.: Responsible for echinococcosis, these parasites may remain asymptomatic until they cause significant complications. Diagnosis often requires imaging techniques like ultrasound or CT scans, along with serological tests.
- Treatment varies based on cyst type and may involve albendazole (400 mg twice daily) or surgical interventions.
- Filariasis: A diverse group of diseases caused by nematode parasites, filariasis can present with various symptoms, including lymphatic dysfunction and skin manifestations.
- Diagnosis involves identifying microfilariae in blood, urine, or skin samples, alongside serological tests.
- Treatment for onchocerciasis involves ivermectin, while lymphatic filariasis may require different management strategies based on clinical presentation.
- Castro GA. Helminths: Structure, Classification, Growth, and Development. In: Baron S, editor. Medical Microbiology. 4th edition. Galveston (TX): University of Texas Medical Branch at Galveston; 1996. Chapter 86. Available from: https://www.ncbi.nlm.nih.gov/books/NBK8282/
- Wakelin D. Helminths: Pathogenesis and Defenses. In: Baron S, editor. Medical Microbiology. 4th edition. Galveston (TX): University of Texas Medical Branch at Galveston; 1996. Chapter 87. Available from: https://www.ncbi.nlm.nih.gov/books/NBK8191/
- https://pharmaceutical-journal.com/article/ld/helminth-infections-diagnosis-and-treatment
- https://www.econtent.in/pacc.in/admin/contents/60_MZO%2011_2020111705391073.pdf
- https://www.biologydiscussion.com/invertebrate-zoology/arthropods/parasitic-adaptations-of-helminths-parasitology/62108
- https://biozoomer.com/2014/09/parasitic-adaptations-in-helminths.html
- https://www.scribd.com/presentation/580714007/Presentation-4
- https://courses.lumenlearning.com/suny-mcc-microbiology/chapter/parasitic-helminths/
- https://musculoskeletalkey.com/helminths-the-basic-facts/