Epi-Fluorescence Microscopy is defined as an optical imaging method, which is employed in laboratories for visualization of fluorescent-labeled samples. The technique is widely described as an essential approach, and it has been repeatedly applied in cell studies, tissue examinations, and even in material analysis.
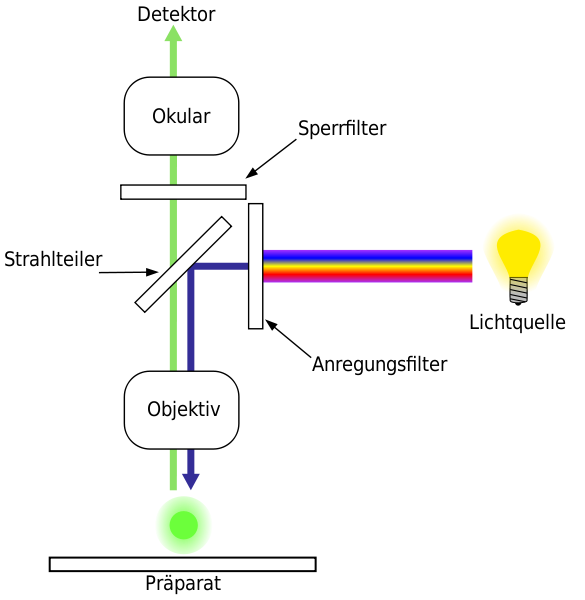
In this method, illumination is delivered from the same side / direction as the objective lens, instead of being provided from underneath as in transmitted light microscopy. A Dichroic Mirror is utilized, and by it the excitation wavelength is reflected toward the specimen, while the emission is allowed to pass into detection pathway. The arrangement is considered efficient, but sometimes background signal is unavoidably produced.12
The Fluorophores are molecules, which have been selected for their inherent property of absorbing light at one wavelength, and emitting at a longer wavelength. Upon excitation, electrons are shifted into a higher energy level, and when return happens to the ground state, photons are released. This emission is then filtered by a barrier filter, so that the unwanted excitation is excluded, although leakage might be still present in some cases.
It is noted that epi-fluorescence microscopy has been described as both simple and complicated. The system is simple in construction, because one objective lens is shared for both illumination and observation, but complicated alignments may be required when different filter sets are used. The principle is straightforward, yet artifacts are often introduced, and the user must be careful, since interpretation is sometimes misleading.
The samples are illuminated from the top/front side, and surface structures are emphasized. This is advantageous, because thin layers or membrane details can be highlighted, although deeper structures are less resolved. It is known that Fluorescence provides strong contrast, which is extremely beneficial in biological imaging.23
Applications are numerous. Proteins are located, nucleic acids are stained, pathogens are detected, and cellular dynamics are followed in real time. The antibodies are conjugated with fluorophores, and immuno-fluorescence assay is developed, which has become a standard diagnostic tool. The method is used in microbiology, genetics, nanotechnology, or in medical sciences, and these areas have been revolutionized by its use.
Nevertheless, limitations are also recognized. Fluorophores are affected by photobleaching, which reduces their brightness with time, and autofluorescence from sample can interfere with clarity. Resolution is ultimately limited by diffraction of light, and therefore molecular dimensions smaller than ~200 nm cannot be resolved.
The Epi-fluorescence Microscopy has been considered as one of the fundamental techniques of modern research / diagnostic imaging. It has been adopted everywhere, and despite drawbacks, it is continuously used. The importance of this technique cannot be overstated, because although alternatives exist, the method still remains as indispensable, which is why it is repeatedly relied upon.1410
What is the Principle of Epi-Fluorescence Microscopy?
The Working Principle of the Epi-fluorescence Microscopy is usually described in passive terms, which is appropriate, because illumination and detection are arranged to occur through the same Objective, and the pathways are caused to overlap deliberately.
Illumination is delivered from the top / same side of observation, and it is guided into the Objective lens (NA, focal length in mm) after passing an Excitation filter (λ_ex in nm), which selects a narrow band of wavelengths. A Dichroic Mirror is then positioned at 45°, and by it the excitation is reflected toward the specimen, while the longer-wavelength light is later transmitted back to the detector, which makes separation possible.
A Specimen containing Fluorophores is prepared at room temperature (RT, ~22 °C) or other controlled conditions (37 °C), and mounting in buffer (PBS, NaCl, pH 7.4) is often performed. The surface of the sample is illuminated from above, so near-surface structures are emphasized, while deeper features are de-emphasized, which is an expected consequence of geometry.
Fluorophores have inherent properties. They absorb shorter-wavelength photons, and they emit longer-wavelength photons (Stokes shift). Their absorption spectra and emission spectra are fixed by chemistry, and the quantum yield belongs to them inherently.
Upon excitation, electrons in the fluorophore are promoted to an excited state, after which relaxation occurs, and photons are released as fluorescence (τ in ns). The emitted photons are allowed to pass through the Dichroic Mirror into the detection path, and a Barrier (emission) filter (λ_em in nm, bandwidth in nm) is placed, which removes residual excitation light, although some leakage may be seen, which is not surprising.
Image formation is produced by the same Objective that provided illumination, and therefore the optical throughput is increased, and collection efficiency is improved, but sensitivity to stray reflections is also increased. The camera (sCMOS) or the eye is presented only with the selected emission band, so contrast is provided strongly by fluorescence, and background is reduced—though not eliminated.67
What are the Essential Parts of an Epi-Fluorescence Microscope?
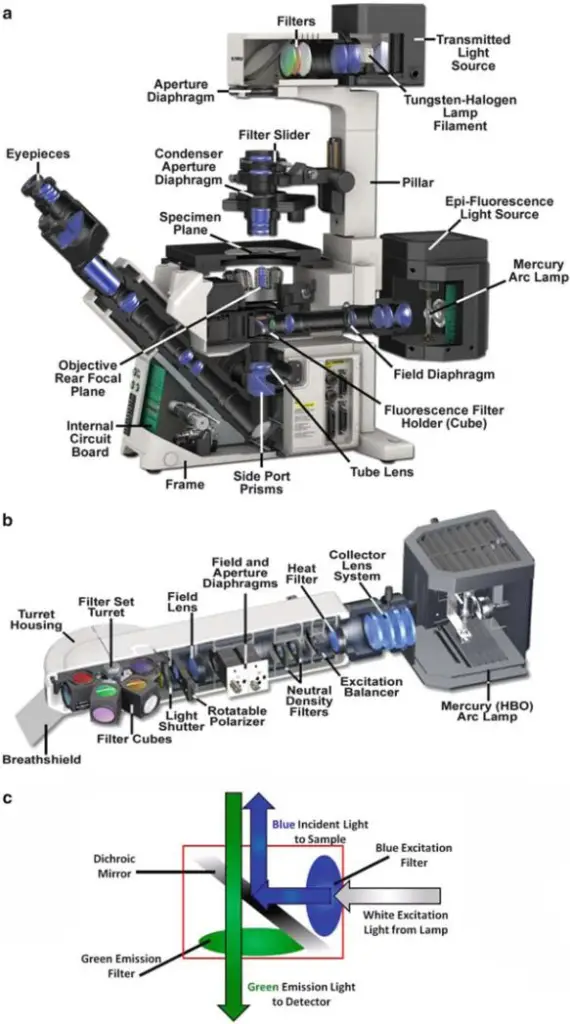
- The Light Source is provided, and it is usually a mercury lamp/ LED lamp, which is required for illumination of the specimen, although at times an other source is used, and it is placed mostly on the back of microscope body.
- An Excitation Filter is used, and by it, only selected wavelength of light is allowed to pass, while unnecessary wavelengths are blocked, which ensures the correct excitation of fluorescent dyes.
- Dichroic Mirror is positioned, and by this mirror, the excitation light is reflected downward toward specimen, but the emission light is permitted to pass through, although sometimes leakage is happening, which is undesirable.
- Objective Lens are mounted, and magnification is inherently provided, as optical property belongs to the lens, and the excitation light is focused onto the sample through them, while the same objective is used to collect emitted fluorescence, so both illumination/collection are performed together.
- Emission Filter is placed, and only the specific wavelength that belongs to fluorescence signal is transmitted, but stray excitation light is blocked, and this filtering step is carefully designed.
- The Eyepiece/Ocular Lens is given, and through it, observer views the magnified fluorescent image, while sometimes a digital camera is attached, for capturing image, which later is analyzed.
- Mechanical Stage is adjusted, and the specimen slide is put upon it, so movement and positioning are enabled, and with stage controls, the sample is moved in X/Y directions, however vibration is causing blurred image, which becomes problematic.
- Focus Knobs are included, and coarse/fine adjustments are made for bringing image into clarity, both knobs are usually located at side, though their exact dimension is sometimes different.
- Filter Cube Housing is installed, and within it, excitation filter, dichroic mirror, and emission filter are arranged together in one cube, so switching between channels is made easier, but alignment issues are possible.
- Body Tube/Optical Path is constructed, and in it, light beams are guided from objective to ocular lens or digital camera, and misalignment in path is often observed, which reduces image clarity.
- Power Supply is connected, and stable electricity is provided, although fluctuations are occurring, which create uneven illumination of the sample.
- Cooling Fans/Heat Sink are attached, because lamp produces high heat (temperature °C), so overheating is prevented with forced-air cooling, but long duration usage is still harmful.
- Digital Camera or Imaging Device is mounted, and fluorescent signals are recorded, image analysis software is linked, but calibration errors are done by user sometimes, which reduce the accuracy.816
How to Prepare Samples for Epi-Fluorescence Microscopy?
- Thick samples (=10 µm depth) are often viewed and observed, although significant background signal is produced due to intense lighting / activation of molecules beyond the focal point.
- The cells are grown in vitro, and they are fixed on the coverslips by incubation, although fixation time and conditions are variable.
- Proteins are incubated in different solutions at different temperatures, and the variations influence stability, which is important.
- Phosphate Buffer Saline (PBS, 1x) is used for rinsing, and rinsing is done three times, but sometimes incomplete washing is occurring.
- A permeabilization is performed with 0.2% Triton X-100 for 5 minutes at room temperature, although the duration can be adjusted, which sometimes give different results.
- Again, rinsing with PBS is repeated for three times / again, and debris or detergent residues are removed although not completely always.
- To prevent the drying, the coverslips are placed in humid environment, which is created by wet Kimwipes in incubation chamber, although drying still may occur.
- The incubation with 100 µL of 20% goat serum is done for 1 hour at room temperature, and this blocking reduce nonspecific binding, which interfere in fluorescence signals.
- Primary antibody is diluted in 5% goat serum at 1:100 dilution, but the concentration is altered depending on antibody type or specificity.
- Coverslips are incubated with 100 µL of primary antibody at room temperature for 1 hour, or sometimes incubation is performed overnight at 4 °C.
- The rinsing step with PBS is performed again / repeated three times, although traces of antibody may still remain, which affect results.
- Secondary antibody conjugated with fluorophore (FITC, TRITC, Alexa Fluor etc.) is diluted in 5% goat serum at 1:500 and stored in the dark, and specificity is tested to minimize nonspecific labeling.
- Incubation of coverslips with 100 µL of secondary antibody is done for 1 hour at room temperature, but overnight incubation should be avoided, because nonspecific binding increases.
- The coverslips are washed with PBS three times, although washing steps sometimes lose some specific signal.
- Mounting medium (≈10 µL) with anti-fade agents (ThermoFisher type) is placed on slide, and coverslip is inverted gently, while pressing is done with cotton swab to avoid bubbles / artifacts.
- The sealing is carried out by Vaseline, Lanolin, and Paraffin mixture (1:1:1), although other sealing compounds are sometimes applied.
- The specimen is preserved in mounting medium which cures and hardens, and storage is done at 4 °C in the dark for weeks, although fluorescence fading may still occur.
- Finally, the prepared sample is ready, and it is placed on the microscope stage for imaging by epi-fluorescence illumination, while emitted signals are collected and observed.918
What is the Step-by-Step Operating Procedure for Epi-Fluorescence Microscopy?
- The microscope is placed on a stable bench, and vibrations are minimized, although minor vibration sometimes persist, which affect image quality.
- Power is applied to the system, and a warm-up period is allowed, because excitation lamp (Mercury, or LED) requires thermal stabilization, which ensures intensity is steady.
- Objectives are inspected, and they are cleaned with lens tissue, while immersion oil (if 100x objective) is applied sparingly, although excess oil may spread.
- A sample slide is mounted on Stage, and the coverslip is oriented upward / towards objective, although incorrect orientation is sometimes made by users.
- Stage clamps are adjusted to secure the slide, while micrometer controls are used to position the specimen, which is important for focal stability.
- Transmitted light illumination is activated first, and the specimen is coarsely focused at low magnification, because coarse focusing reduce the risk of collision with objective.
- The appropriate excitation filter cube (DAPI, FITC, TRITC etc.) is selected, and the cube is inserted into turret, although mis-selection of cube will produce wrong excitation.
- The fluorescence Lamp shutter is opened, and excitation light is directed via dichroic mirror, while the emission path is aligned for detector, which is essential for signal collection.
- Fine focus is adjusted slowly, until a sharp fluorescence image is observed, and small stage drifts are compensated for, although drift may still occur.
- Exposure time and camera gain are optimized, and neutral density filters are interposed to limit illumination intensity / prevent photobleaching, which prolong lamp and fluorophore life.
- Detector (CCD, or CMOS) parameters are set, and test frames are acquired to check signal-to-noise, while histogram checks are performed to avoid saturation.
- Sequential acquisition is programmed when multiple fluorophores are present, and filter sets are changed between channels to reduce bleed-through, which otherwise confound quantification.
- Background is estimated and subtracted in software, and raw images are archived, although raw file sizes can be large which stress storage.
- Lamp shutter is closed between exposures, and the excitation source is turned off when not in use, because lamp life is extended by such practice.
- Objective immersion oil residues are removed immediately after imaging, and the slide is taken off the stage, although sometimes residue remain which degrade optics.
- Data files are saved, and backups are created on local and remote media, while checksums or integrity checks are recommended to detect corruption.
- Routine calibration is performed using fluorescent beads, and system alignment is verified periodically, because quantitative imaging depends on stable optics.
- Objectives consist of multiple glass lens elements that are designed to collect emitted photons efficiently, and this is an inherent property of the optics.
- Dichroic mirrors reflect excitation, and they transmit emission, which provides the required spectral separation for channel discrimination.
- Safety procedures are followed, and eye protection is recommended whenever high intensity lamps are used, although interlocks sometimes fail.
- The microscope is powered down following manufacturer recommended sequence, and dust cover is placed, while room humidity is controlled to minimize contamination.
- Ensure alignment before data acquisition.
- Close the shutter when imaging is completed.
- Record experimental metadata (exposure, filters, objectives) in the image log, and keep a brief notes file, which facilitate reproducibility.14
What are the Key Applications of Epi-Fluorescence Microscopy?
- The Epi-fluorescence Microscope are applied in Cell biology, and proteins are observed by fluorescent tagging, although the fluorophore are fading quickly which decrease clarity of image, and repetition of experiment is sometimes needed for reliability.
- The DNA and the RNA are detected by fluorescent probes, and the labeling is done in situ / in vitro, although nonspecific binding are happened, which interfere with the correct interpretation of hybridization experiments.
- The localization of proteins inside Nucleus or in cytoplasmic compartment are studied, and images is captured, although overlapping signals are frequently noticed which complicates the conclusions.
- Cellular dynamic processes are monitored, and time-lapse imaging is performed, although the living specimen are damaged by photo-toxicity, and this effect is reduced only when temperature (37 °C) and humidity are carefully controlled, which not always is successful.
- In microbiology the pathogens detection are carried out, and immunofluorescence methods are applied, although cross-reactivity between antigen–antibody is frequent, which results into false signals.
- Organelles such as mitochondria, lysosome, and Golgi Apparatus are stained, and the structure are analyzed, although diffusion of dye into non-target areas are also happening, which cause morphological changes that are misleading.
- Protein interaction are investigated, and FRET (Förster Resonance Energy Transfer) signals is recorded, which indicate close molecular distances, although bleaching of acceptor fluorophore are frequently observed, which complicates the interpretation.
- Co-localization are performed in multi-channel acquisition, and overlapping of emission signals is done / compared, although mechanical drift of filter cube are disturbing alignment, which reduces accuracy.
- Quantitative measurement of fluorescence are attempted, and the expression levels are compared, although intensity are dependent on lamp stability, exposure time, fluorophore concentration, and detector efficiency, which produce inconsistent results.
- Live cell motility are tracked, and frames are recorded sequentially, although the photo-damage are reducing viability, and experiment is stopped earlier than planned.
- In the environmental Science, microbial population are studied in soil particles and aquatic samples, although autofluorescence from organic material is present, which interfere with clarity of observation.
- The medical diagnostics are performed, and tissue biopsy are examined by immunofluorescence staining, although artifacts from fixation are distorting tissue structure, which mislead analysis.
- Drug delivery studies are carried out, and nanoparticles labeled with fluorescent marker are traced, although aggregation of marker are giving false appearance of distribution.
- Calcium dynamics are observed, and fluorescent indicators (Fluo-4, Fura-2) are used, although calibration procedure are complicated, which result in semi-quantitative rather than absolute measurements.
- Plant tissues are analyzed, and chlorophyll autofluorescence are detected, although the strong background fluorescence are interfering with external probes.
- Transcription factor localization are examined, and the compartmentalization in nucleus or cytoplasm is reported, although protein redistribution during fixation are influencing the outcomes.
- Biofilm structure are observed, and bacteria are stained with viability dyes, although fading of fluorescence are occurring during imaging, which reduce the accuracy, and biofilm detachment from slides are sometimes occurring.
- The educational use are common, and microscopy principles are demonstrated in teaching laboratory, although mishandling by students are producing unfocused images or incorrect filter cube selection.515
What are the Main Advantages of Epi-Fluorescence Microscopy?
- The High Sensitivity of Fluorescent molecules is utilized, and therefore even small structures are detected with higher efficiency, although in some cases, the detection may be influenced by background noise.
- A Specificity of visualization is provided because fluorescent labeling is performed, and hence a particular component, protein, or organelle is distinguished from surrounding matter.
- The Technique is widely applied for the study of living cells/tissues, since fluorescence tagging is accomplished without always requiring destructive processing, which ensures observation in real time manner.
- The Multiple fluorophores can be used simultaneously, and because of this reason, a multiplexed analysis of different structures is facilitated, while overlap between emission spectra sometimes is causing difficulties.
- An Improved contrast is offered, because the emitted fluorescence light is being collected from the sample itself, and thereby unwanted illumination from other directions is reduced/blocked.
- High Resolution Imaging is enabled when proper optics, filters, and detectors are used, and therefore very fine structural details are visualized, even though photobleaching is frequently occurring in longer observations.
- Non-invasive procedures are supported, and so the natural physiological states of cells are maintained during the observations, although repeated illumination may influence its viability.
- The Rapid image acquisition is made possible, since fluorescent signals are inherently strong, and images are captured in relatively short exposures, which is especially beneficial for dynamic or fast biological processes.
- Wide Range of fluorophores is commercially available, and by that, flexibility is provided to researchers in selection of dyes/markers, although sometimes autofluorescence of the sample is producing confusion.
- It is Established as a standard tool in cell biology, microbiology, and medical diagnostics, since reproducibility of results is supported, and compatibility with advanced digital imaging systems (CCD, CMOS) is achieved.
- Structural as well as functional informations are obtained together, because fluorescent probes are designed not only to localize but also to measure processes like pH, ion concentration (Ca²⁺), or membrane potential.
- The Technique is adapted easily into modern advanced methods, for example Confocal microscopy or Super-resolution approaches, and thus greater depth of field and finer resolution are extended into practice.13
What are the Limitations of Epi-Fluorescence Microscopy?
- A Significant photobleaching is frequently observed, and the fluorescent molecules are degraded under continuous illumination, which reduce the duration for meaningful observations.
- The High background signal is generated because autofluorescence of biological samples is often present, and this interference is complicating the interpretation of data.
- A Problem of phototoxicity is encountered, since cells/tissues are damaged by repeated exposure to excitation light, although protective measures are sometimes applied for minimizing.
- The Depth of imaging is limited, because excitation and emission light are scattered and absorbed within thicker samples/tissues, which make accurate visualization difficult.
- Resolution is restricted by the diffraction limit of light, although certain modifications are proposed, and thus the finest nanoscale structures are not easily observed in this technique.
- Overlapping of emission spectra from multiple fluorophores is producing a cross-talk, and therefore precise separation of signals is often difficult, even though filter sets are employed.
- The Stability of fluorescent dyes is variable, since some are fading rapidly, and others show batch-to-batch inconsistency, which is giving rise to reduced reproducibility of experiments.
- Sample preparation is sometimes complex/tedious, and additional steps of labeling are required, although these extra treatments are introducing possible artifacts into the observation.
- Quantification of fluorescence intensity is not always accurate, because variation in staining efficiency, illumination intensity, or detector sensitivity is contributing to inconsistent results.
- A Limitation of cost is noted, since specialized filters, optics, and fluorophores are expensive, and accessibility of resources is thereby restricted in some laboratories.
- The Data interpretation is challenging, because non-specific binding of fluorescent labels is occurring, which lead to ambiguous outcomes that complicates the analysis process.
- Rapid bleaching of fluorophores during high-resolution or time-lapse studies is resulting in poor continuity of images, although this effect is slightly reduced by use of anti-fade reagents.
- The Technique is affected by a lack of depth discrimination, because signals from out-of-focus planes are collected, and image clarity is consequently compromised.
- Limitations in compatibility with live-cell conditions are often noticed, since high-intensity excitation light is interfering with natural physiological states of the cell/organism.
What Safety Precautions Should You Follow When Using Epi-Fluorescence Microscopy?
- The Direct exposure to intense excitation light should be avoided, because harmful ultraviolet (UV) radiation is emitted, which may damage eyes or skin during careless handling.
- Protective eyewear is required to be worn, and the goggles with proper optical density are recommended, although sometimes users forget and unnecessary risk is taken.
- The Use of appropriate shielding and barriers around the light path is ensured, since scattered light is causing hazards, and so preventive steps must be adopted.
- A Careful handling of fluorescent dyes/chemicals is necessary, because some of them are toxic, mutagenic, or carcinogenic, which demand that gloves and protective clothing be worn.
- Ventilation of the workspace is to be maintained, as fumes from certain solvents or fixatives (formaldehyde, ethanol, acetone) are hazardous, and inhalation exposure should be minimized.
- The Sample preparation areas should be kept separated from the imaging area, and therefore contamination risk is reduced/controlled, though lapses in practice sometimes are happening.
- A Regular inspection of filters, light sources, and optical components is required, because degradation of these parts is leading to safety risks and inaccurate performance.
- The Intensity of illumination must be minimized whenever possible, since both photobleaching and phototoxic effects are worsened under high light conditions, although experiments are often demanding brighter settings.
- Electrical safety should be ensured, and all power cords, sockets, and connections are checked for damages, which otherwise might result in shocks or fire hazard.
- The Microscope should be placed on stable surfaces, because vibrations or accidental displacement is causing harm to the equipment and operator at same time.
- Proper training of personnel is to be provided, as untrained individuals are mishandling instruments, and this mishandling is resulting into unsafe and non-standardized practices.
- The Disposal of used fluorescent stains, slides, and chemicals must be carried out following institutional biosafety guidelines/regulations, although negligence is sometimes seen in laboratory routines.
- A Calibration and alignment of optical systems should be performed regularly, because misaligned optics are not only reducing image quality but also increasing exposure risks.
- The Emergency procedures for accidental exposure, spillage, or equipment failure are to be established, and clear instructions should be displayed/communicated to all users.
- Long exposures during image acquisition must be carefully controlled, since both human safety and sample viability are compromised, although repeated experiments often encourage longer imaging sessions.1117