What is the Electrofusion of Plant Cells?
- Electrofusion of plant cells represents a significant advancement in the field of plant biotechnology, particularly in genetic modification and plant breeding. This technique facilitates the fusion of isolated protoplasts—plant cells that have had their cell walls removed—derived from different species. As a result, it offers a valuable system for creating hybrid plants that can exhibit desirable traits from both parent species.
- The electrofusion process was developed relatively recently and requires specialized equipment that is becoming increasingly available to researchers. Unlike traditional methods that utilize chemical agents to induce protoplast fusion, electrofusion leverages electrical fields. This approach presents several advantages, particularly in achieving higher fusion frequencies. Studies indicate that the fusion rates attained through electrofusion can be up to ten times greater than those achieved using chemical stimulation methods.
- One of the most notable benefits of electrofusion is the minimization of toxic chemical stimulants, which can compromise protoplast viability. In electrofusion, the disturbances to the cell membrane are localized to the areas of membrane contact, thereby preserving the integrity of the protoplasts. Moreover, this technique allows for real-time microscopic monitoring of fusion events. Researchers can meticulously analyze the effects of various electrical parameters, thus optimizing the conditions for successful cell fusion.
- Additionally, the manipulation of electrodes in the electrofusion apparatus enables precise control over the fusion process. This level of control permits researchers to better define the parentage of the resulting hybrid cells, which was not achievable with earlier methods. For instance, this protocol has been employed in efforts to enhance disease resistance in crop species. A case in point is the endeavor to bolster disease resistance in celery varieties, which are particularly vulnerable to late blight caused by the fungus Septoria apiicola.
- The late blight disease is characterized by the direct penetration of leaf surfaces by the fungus, followed by its proliferation between plant cells. The resulting infection manifests as brown or black spots on the leaves, significantly affecting crop yield and quality. Conventional breeding approaches have struggled to enhance resistance levels in susceptible celery varieties, necessitating the exploration of innovative solutions like electrofusion.
Materials Required for Electrofusion
Electrofusion of plant cells involves a variety of materials essential for the successful execution of the technique. The following is a comprehensive list of the materials required, each with a detailed explanation of its function in the electrofusion process:
- Solution for Seed Surface Sterilization:
- A 20% solution of Domestos (or any commercial bleach) is used for sterilizing seed surfaces. This step is crucial to eliminate contaminants that could interfere with subsequent culture processes.
- Germination Agar (GA):
- The germination medium consists of Murashige and Skoog (MS) salts supplemented with 3% sucrose and 1% agar. This combination supports seed germination by providing essential nutrients and a solid matrix for seedling establishment.
- Callus Induction Medium (CIM):
- This medium is prepared using MS salts, supplemented with 3% sucrose, 0.5 mg/L of 2,4-dichlorophenoxyacetic acid (2,4-D), and 0.6 mg/L of kinetin, along with 1% agar. CIM promotes the formation of callus tissue from explants, which is a mass of undifferentiated cells that can be used for further manipulation.
- Cell Suspension Medium (CSM):
- CSM is formulated with MS salts, 3% sucrose, 0.5 mg/L of 2,4-D, and 0.6 mg/L of kinetin. This medium is essential for maintaining protoplasts in a suspension, facilitating their division and growth before fusion.
- Solution for Plasmolysis Treatment:
- A 0.6M mannitol solution is used to induce plasmolysis in protoplasts. This process is necessary to make the protoplasts more receptive to fusion by creating a suitable osmotic environment.
- Lytic Mixture:
- This mixture contains specific enzymes in 0.6M mannitol, freshly prepared for each use. It should be filtered sterilized and adjusted to a pH of 5.6 as needed. The components of the lytic mixture vary based on the source material:
- For celery, it includes 0.1% Pectolyase Y23 (from R.W. Unwin & Co. Ltd.) and 2% Celluysin (from Cambridge Bioscience).
- For lovage, the mixture consists of 0.1% Pectolyase Y23 and 3% Meicellase (from R.W. Unwin & Co. Ltd.). These enzymes facilitate the degradation of cell walls, enabling protoplast isolation.
- This mixture contains specific enzymes in 0.6M mannitol, freshly prepared for each use. It should be filtered sterilized and adjusted to a pH of 5.6 as needed. The components of the lytic mixture vary based on the source material:
- Protoplast Washing Solution:
- A washing solution made of 0.6M mannitol containing 2 mM calcium chloride is utilized to wash isolated protoplasts. This step helps to remove any residual enzymes or debris, ensuring high viability for subsequent fusion.
- Medium for Culture of Electro-fused Protoplasts (EPM):
- The electro-fusion medium (EPM) consists of NTK medium devoid of ammonium salts, but supplemented with 100 mM calcium chloride, 0.5 mg/L of 2,4-D, and 0.6 mg/L of kinetin. This specialized medium provides optimal conditions for the culture and development of protoplasts after electrofusion, supporting their growth and division.
Electrofusion Protocol
The following detailed methodology outlines the protocols for the growth of sterile seedlings, cell suspensions, protoplast isolation, protoplast fusion, and the culture of electrofused protoplasts.
- Growth of Sterile Seedlings
- To initiate the process, seeds must be surface sterilized in a solution of 20% Domestos for 12 minutes.
- Following sterilization, seeds should be washed three times in sterile distilled water to remove any remaining disinfectant.
- Aseptically place the seeds onto germination agar (GA) in Universal vials.
- The vials are then incubated at 4°C in darkness for 5 days, followed by a shift to 22°C under light for a duration of 8 weeks for Apium graveolens (celery, variety Celebrity) or 12-16 weeks for Levisticum officinale (lovage). Optimal conditions for germination include high light and humidity.
- Young leaf material harvested from these seedlings is suitable for protoplast isolation.
- Growth of Cell Suspensions
- Petiole explants, measuring 1 cm, from the laboratory-grown seedlings are placed onto callus induction medium (CIM).
- These explants are incubated at 22°C under a 12-hour light/dark cycle for 8 weeks.
- After this period, the callus tissue should be subcultured and incubated for an additional 4 weeks.
- Subsequently, the callus tissue is transferred to 50 mL of cell suspension medium (CSM) within 250-mL flasks. The cultures are shaken at 100 rpm while maintained at 22°C under the same light/dark regime.
- Subculturing occurs every 4 weeks, with 7-day-old suspension culture material used for protoplast isolation.
- Isolation of Protoplasts
- For protoplast isolation, finely chop young leaf material, approximately 0.5 g fresh weight, and plasmolyze in a 0.6 M mannitol solution for 10 minutes.
- Alternatively, 7-day-old cell suspension cultures can be utilized. Allow the suspension cultures to settle for 10 minutes, and then use the cells from 10 mL of the settled suspension.
- Transfer the selected cells to a lytic mixture (10 mL) appropriate for the tissue type and incubate at 22°C with gentle shaking. The incubation time varies: 3 hours for celery mesophyll tissue, 5 hours for lovage mesophyll tissue, or overnight for celery cell suspension material.
- After incubation, protoplasts are separated from the lytic mixture by filtering through a 70 µm nylon net, followed by centrifugation at 100 g for 2 minutes to remove cell debris.
- The resultant pellet is resuspended in a protoplast washing solution (PWS) and subjected to additional washes in PWS, yielding an estimated 5 x 10^6 protoplasts per gram of mesophyll tissue.
- Protoplast Fusion
- A Zimmermann Cell Fusion System from GCA Corporation is employed to generate the necessary electric fields for protoplast fusion. This protocol utilizes a flat fusion chamber equipped with two fine, wired electrodes arranged in parallel on a polyethylene slide, having a 17 µm capacity. It is compatible with larger capacity 200 µL helical chambers as well.
- First, protoplasts are isolated and resuspended in a washing solution (PWS), mixing equal quantities from the species intended for fusion to achieve a final concentration of 10^5 cells/mL.
- The fusion chamber is filled with this protoplast mixture.
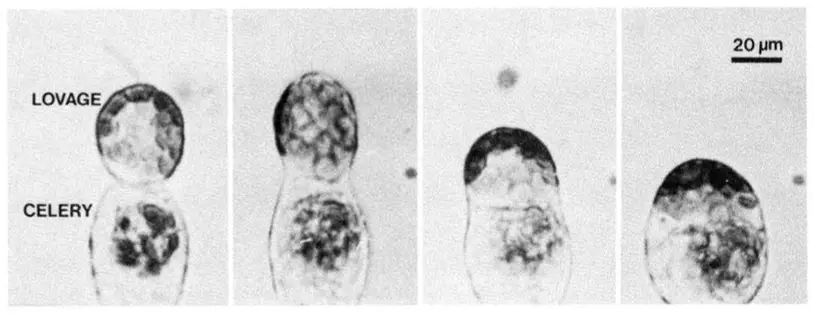
- An alignment frequency of 2 MHz and an alignment voltage of 5 V are applied, causing protoplasts to align at the electrodes, forming “pearl chains” of varying lengths.
- The percentage of protoplasts aligning and forming these chains is determined, observing the extent of membrane contact.
- Next, a fusion voltage of 30 V is applied in two pulses of 90 µs duration, separated by 1-second intervals. During this phase, protoplasts align and fuse upon exposure to short DC pulses. After fusion, the protoplasts round up over several minutes.
- Finally, the fused protoplasts are transferred to a culture medium.
- Culture of Electro-Fused Protoplasts
- To culture the electrofused protoplasts, they should be washed into Universal vials using culture medium (EPM) at a 1:5 ratio.
- The protoplasts are cultured as sitting drops (0.1-0.2 mL) in 6 cm diameter Petri dishes, typically with seven drops per dish, and sealed with Labfilm at 22°C.
- Control samples, which are not subjected to the electrofusion process, should also be included for comparison.
- After 3 days, the drops are examined microscopically to detect any cell division, with Evans’ Blue vital dye employed to assess protoplast viability.
- Any dividing cells are subsequently transferred to callus induction medium (CIM) for further cultivation.
Important Notes
The following notes provide essential guidelines and observations to enhance the effectiveness of the electrofusion process.
- To maximize protoplast viability:
- Minimize lytic incubation times: Prolonged exposure to lytic mixtures can lead to protoplast lysis, resulting in reduced viability. Therefore, it is crucial to limit the time spent in these solutions while maintaining effective enzyme activity.
- Utilize low enzyme concentrations: Using lower concentrations of lytic enzymes is recommended whenever feasible. This approach reduces the likelihood of protoplast degradation while still allowing for efficient protoplast isolation.
- Regarding the quality of protoplasts:
- High-quality protoplasts exhibit better regeneration potential. The isolation method employed is designed to yield large, spherical protoplasts while minimizing the presence of cellular debris, which can hinder subsequent fusion and growth.
- Identifying fused products:
- Differentiating between protoplasts from various sources can facilitate the identification of fusion products. For instance, lovage mesophyll protoplasts are richer in chloroplasts and appear “greener” than celery mesophyll protoplasts, which lack chloroplasts entirely and are easily distinguishable.
- Fusion efficiency statistics:
- In mixtures of celery and lovage protoplasts, a fusion frequency of 40% can be achieved, with approximately 20% of the resulting fused products identified as heterokaryons. This information underscores the potential for generating hybrid cells with diverse genetic backgrounds.
- Protoplast size and alignment:
- Small protoplasts necessitate higher field strengths and frequencies to effectively form pearl chains during the electrofusion process. Conversely, larger protoplasts typically align at the electrodes more readily.
- The density of protoplasts in the fusion chamber significantly impacts the formation of pearl chains. When the protoplast density exceeds 10^5 protoplasts/mL, there is an observable increase in the length of these chains, indicating a higher likelihood of successful fusion events. Ideally, one-to-one protoplast pairing at electrodes enhances fusion efficiency. Notably, in the lovage/celery mixtures, 50% of pearl chains comprised both protoplast types.
- Voltage requirements:
- Smaller protoplasts require higher direct current (DC) voltages to facilitate fusion. In situations where there is a considerable size disparity between the protoplasts to be fused, treatment with Pronase E (Pronase, Type XXV, Sigma Co. Ltd.) can stabilize larger protoplasts.
- Incubation at 30°C for 30 minutes to 1 hour with a concentration of 1 mg/mL Pronase E has been shown to enhance the fusion frequency of more recalcitrant materials.
Advantages of Electrofusion method
Electrofusion offers several advantages in plant biotechnology and genetic engineering, particularly in the fusion of protoplasts. Below are some key benefits of this technique:
- Increased Fusion Efficiency: Electrofusion significantly enhances the likelihood of protoplast fusion compared to traditional methods. The application of electric fields facilitates the alignment and fusion of protoplasts, resulting in higher fusion frequencies.
- Hybrid Plant Development: This method allows for the creation of hybrids between different plant species or varieties, potentially leading to new plants with improved traits, such as enhanced disease resistance, greater yield, or better adaptability to environmental stresses.
- Targeted Genetic Recombination: Electrofusion enables the combination of genetic material from distinct sources, allowing researchers to introduce desirable genes from one species into another. This targeted approach can accelerate the development of plants with specific characteristics.
- Minimized Contamination Risk: The closed system used in electrofusion reduces the risk of microbial contamination during the fusion process. This is particularly important for maintaining the integrity of plant tissues and cultures.
- Versatility in Protoplast Sources: Electrofusion can be applied to a variety of plant tissues and species, providing flexibility in experimental design. This versatility allows researchers to explore numerous combinations of plant materials.
- Reproducibility and Standardization: The electrofusion protocol can be standardized and reproduced across different laboratories, facilitating comparisons between studies and enhancing the reliability of results.
- Preservation of Cell Integrity: The process is designed to minimize damage to protoplasts, preserving their cellular structures and functions. High-quality protoplasts are more likely to regenerate and grow into viable plant cells.
- Potential for Genetic Improvement: By facilitating the transfer of genetic material between plants, electrofusion has the potential to contribute significantly to the development of new varieties with improved agronomic traits, which can benefit agriculture and food production.
- Reduction of Somatic Embryogenesis: Electrofusion can reduce the need for somatic embryogenesis, which is often a lengthy and complex process. Instead, it allows for more direct methods of producing viable plant hybrids.
Limitations of Electrofusion method
While electrofusion is a powerful technique in plant biotechnology, it also has several limitations that researchers must consider. Below are some of the key drawbacks associated with the electrofusion method:
- Equipment Requirements: The technique requires specialized equipment, such as electrofusion chambers and power supplies. This can be a barrier for some laboratories, particularly those with limited resources or funding.
- Optimization Challenges: Achieving optimal conditions for electrofusion can be complex and may require extensive experimentation. Factors such as electric field strength, duration of exposure, and protoplast density need to be finely tuned, which can be time-consuming.
- Variable Fusion Efficiency: The efficiency of protoplast fusion can vary widely depending on several factors, including the plant species involved, protoplast size, and the conditions used during the fusion process. This variability can make it difficult to predict outcomes consistently.
- Cell Viability Concerns: Although electrofusion is designed to preserve protoplast viability, there remains a risk of cell damage during the process. Excessive electric field strength or prolonged exposure can lead to reduced viability and compromised regeneration potential.
- Limited Scale: While electrofusion works well for small-scale experiments, scaling up the process for commercial applications or larger plant breeding programs can be challenging. The current methods may not be easily adaptable for large-volume production.
- Potential for Genetic Instability: Fusion events may lead to genetic instability in the resulting hybrid cells, which can manifest as phenotypic variations or loss of desirable traits. This instability can complicate the breeding process and affect the reliability of the resulting plants.
- Regulatory Challenges: The use of electrofusion to create genetically modified organisms (GMOs) may face regulatory hurdles in some countries. Navigating these regulations can be time-consuming and may limit the adoption of electrofusion-derived plants.
- Limited Understanding of Mechanisms: Despite its effectiveness, the underlying mechanisms of electrofusion and how different variables affect the process are not fully understood. This lack of understanding can hinder further optimization and application of the technique.
- Complexity of Hybridization: While electrofusion allows for hybridization between different species, the resulting hybrids may not always possess the desired traits or may exhibit reduced fitness in certain environments.
Uses of Electrofusion method
The electrofusion method is utilized in various applications within plant biotechnology and related fields. Below are some of the prominent uses of this technique:
- Creation of Hybrid Plants: Electrofusion is extensively used to produce hybrids by fusing protoplasts from different plant species or varieties. This method allows for the combination of desirable traits from multiple sources, leading to plants with improved characteristics, such as increased yield, disease resistance, or enhanced nutritional value.
- Genetic Engineering: The technique facilitates the introduction of specific genes into plant cells, allowing for targeted genetic modifications. Researchers can create plants that express new traits, such as herbicide tolerance or enhanced flavor, by combining protoplasts with different genetic backgrounds.
- Cell Line Development: Electrofusion is employed to develop cell lines that can be used for research or commercial purposes. This includes the production of valuable secondary metabolites or proteins through the fusion of specialized plant cells.
- Improvement of Crop Varieties: By creating hybrids with superior traits, electrofusion contributes to the development of improved crop varieties. This can lead to better performance in terms of growth rate, pest resistance, and environmental adaptability.
- Plant Breeding Programs: Electrofusion serves as a valuable tool in modern plant breeding programs, enabling the rapid development of new varieties. It accelerates the breeding process by allowing for the combination of diverse genetic material without the limitations of traditional crossbreeding.
- Regeneration of Somatic Embryos: The technique is used to generate somatic embryos from fused protoplasts, which can subsequently develop into whole plants. This is particularly useful for producing genetically uniform plants.
- Research in Plant Physiology: Electrofusion is used in experimental studies to understand cell interactions and developmental processes. By analyzing the behavior of fused protoplasts, researchers can gain insights into cellular mechanisms and gene expression.
- Study of Intercellular Communication: The fusion of protoplasts allows researchers to investigate intercellular signaling and communication between different plant cells. This can help elucidate the roles of various signaling molecules in plant development.
- Conservation of Rare or Endangered Species: Electrofusion can be used to facilitate the conservation of rare or endangered plant species by enabling hybridization and the introduction of genetic diversity. This may help improve the resilience of these species in changing environments.
- Production of Novel Genotypes: The method enables the generation of novel genotypes that may not occur naturally, providing opportunities for scientific exploration and the development of unique plant characteristics.
- Donovan, A., Isaac, S., & Collin, H. A. (n.d.). Electrofusion of Plant Cells. Plant Cell and Tissue Culture, 373–380. doi:10.1385/0-89603-161-6:373
- Donovan, A., Isaac, S., Collin, H.A. (1990). Electrofusion of Plant Cells. In: Pollard, J.W., Walker, J.M. (eds) Plant Cell and Tissue Culture. Methods in Molecular Biology™, vol 6. Humana Press. https://doi.org/10.1385/0-89603-161-6:373
- Van Wert SL, Saunders JA. Electrofusion and electroporation of plants. Plant Physiol. 1992 Jun;99(2):365-7. doi: 10.1104/pp.99.2.365. PMID: 16668891; PMCID: PMC1080468.
- Wert, Sally & Saunders, James. (1992). Electrofusion and Electroporation of Plants. Plant physiology. 99. 365-7. 10.1104/pp.99.2.365.
- https://www.tutorialspoint.com/protoplast-fusion-methods-and-mechanism
- https://www.biologydiscussion.com/plants/protoplasts-plants/protoplast-fusion-meaning-methods-and-its-mechanisms/26481
- https://www.biologydiscussion.com/plants/protoplasts-plants/fusion-of-protoplast-2-methods-biotechnology/71829
- https://experiments.springernature.com/articles/10.1385/0-89603-328-7:181
- https://www.vubhb.cz/Userfiles/Files/pdf/procedure_of_protoplast_electrofusion.pdf