- Cryptococcus neoformans is an encapsulated yeast of the order Tremellomycetes and an obligate aerobe that can survive in both plants and animals.
- The teleomorph of this organism is a filamentous fungus formerly known as Filobasidiella neoformans. As yeast, it is frequently discovered in bird excrement.
- The fungus Cryptococcus neoformans can infect immunocompetent as well as immunocompromised hosts.
- Since its initial description in 1895, Cryptococcus neoformans’s nomenclature has been revised numerous times. Previously, it contained both C. neoformans var. neoformans and C. neoformans var. grubii.
- C. neoformans var. gattii was later classified as a separate species, Cryptococcus gattii. The most recent system of classification divides these varieties into seven species.
- C. neoformans refers to C. neoformans var. grubii. Cryptococcus deneoformans is the new species designation for the former C. neoformans var. neoformans. C. gattii is composed of five distinct species.
- K.J. Kwon-Chung, who obtained cultures of Filobasidiella neoformans by crossing strains of the yeast C. neoformans, first described the teleomorph in 1975. She was able to observe basidia resembling those of the genus Filobasidium, hence the naming of the new genus Filobasidiella.
- As a result of modifications to the International Code of Nomenclature for algae, fungi, and plants, the practice of giving distinct names to teleomorph and anamorph forms of the same fungus was discontinued, and Filobasidiella neoformans is now considered a synonym of Cryptococcus neoformans.
Characteristics of Cryptococcus neoformans
- Typically, Cryptococcus neoformans is a yeast (unicellular) that replicates by budding. It produces hyphae during mating and basidiospores at the terminal of the hyphae prior to spore production.
- Under host-relevant conditions, such as low glucose, serum, 5% carbon dioxide, and low iron, the cells generate a polysaccharide capsule.
- C. neoformans may be difficult to identify in Gram-stained smears of purulent exudates due to the presence of a large gelatinous capsule that precludes definitive staining of the yeast-like cells.
- In such stained preparations, it may appear as either round cells with Gram-positive granular inclusions on a pale lavender cytoplasmic background or as Gram-negative lipoid bodies.
- C. neoformans, when produced as a yeast, has a prominent capsule composed primarily of polysaccharides. The India ink stain is used to easily visualize the capsule in cerebral spinal fluid under a microscope.
- The ink pigment particles do not enter the capsule surrounding the spherical yeast cell, resulting in a zone of clearance or “halo” around the cells.
- This enables the rapid and straightforward identification of C. neoformans. Rarely are unusual morphological forms observed.
- To identify C. neoformans in tissue, mucicarmine stain specifically stains the polysaccharide cell wall. Cryptococcal antigen extracted from cerebrospinal fluid is believed to be the most sensitive test for diagnosing cryptococcal meningitis, although it may be unreliable in HIV-positive patients.
- 2005 marked the publication of the first genome sequence for a strain of C. neoformans (var. neoformans; now C. denitrificans).
- According to studies, colonies of C. neoformans and related fungi growing on the smoldering reactor of the Chernobyl nuclear power plant may be able to use the radiation’s energy for “radiotrophic” development.
Habitat of Cryptococcus neoformans
- Cryptococcus neoformans is a type of yeast that is commonly found in the environment, particularly in soil contaminated with bird droppings. It is also commonly found in other organic materials such as decaying wood and leaves, as well as in stagnant water and dust.
- C. neoformans is known to thrive in a variety of habitats including bird nests, especially those of pigeons, as well as in the vicinity of bat roosts. The fungus can also be found in a variety of other animals including cats, dogs, and livestock.
- It is important to note that while C. neoformans is commonly found in the environment, not all strains are pathogenic to humans. In fact, most people who come into contact with the fungus will not become ill. However, people with weakened immune systems, such as those with HIV/AIDS or those undergoing cancer treatment, are at an increased risk of developing cryptococcosis, a potentially serious fungal infection caused by C. neoformans.
Morphology of Cryptococcus neoformans
- Cryptococcus neoformans is a spherical-shaped yeast that typically ranges in size from 4 to 20 micrometers in diameter. Under the microscope, C. neoformans appears as round to oval-shaped cells with a thick polysaccharide capsule that surrounds the cell wall. The capsule is a hallmark feature of this species and is important in its pathogenicity.
- The capsule is composed of polysaccharides, including glucuronoxylomannan (GXM), and provides protection against host immune defenses. The capsule is also responsible for the characteristic halo appearance seen when the yeast is stained with India ink, a diagnostic test used in the laboratory.
- The cell wall of C. neoformans is composed of chitin, glucan, and mannoproteins. The cytoplasm of the yeast is filled with small vacuoles, which can be visualized with certain stains. The yeast reproduces asexually by budding, and the daughter cells can remain attached to the mother cell, forming chains of cells called pseudohyphae.
- Overall, the unique morphology of C. neoformans, including its thick polysaccharide capsule, helps it evade host immune defenses and contributes to its ability to cause disease in susceptible individuals.
Cultural characteristics of Cryptococcus neoformans
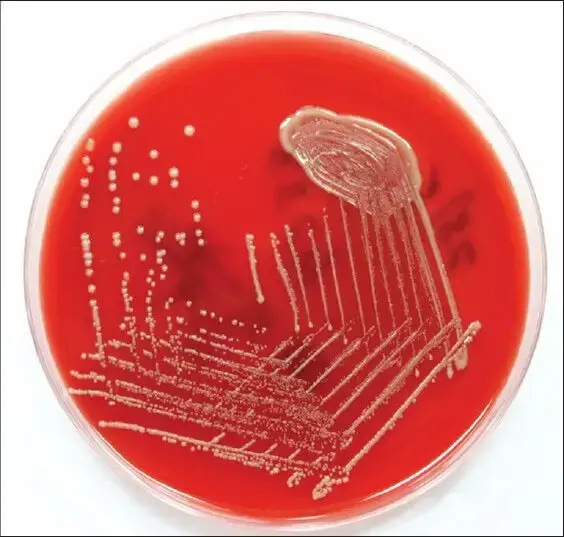
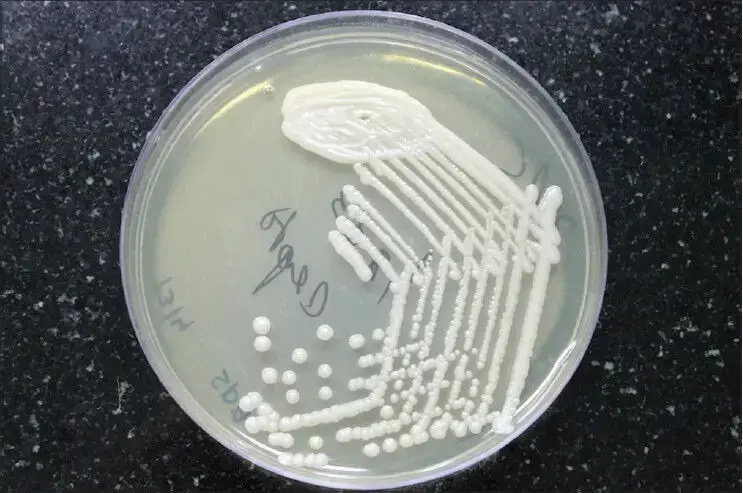
- Cryptococcus neoformans can be cultured on a variety of agar media, including Sabouraud agar, which is commonly used for fungal cultures. On Sabouraud agar, C. neoformans typically appears as smooth, mucoid colonies with a cream to light brown coloration. The colonies can range in size from 2 to 5 millimeters in diameter, depending on the strain and the conditions of the culture.
- C. neoformans is an obligate aerobe and requires oxygen for growth. The optimal temperature for growth is around 30°C, although it can grow at temperatures ranging from 20°C to 37°C. The yeast can also grow at acidic pH levels, typically between 4.5 and 7.0.
- C. neoformans can also be identified using various biochemical tests. For example, the yeast can produce urease, which can be detected using a urease test. It can also produce melanin, which can be detected using melanin production tests.
Life Cycle of Cryptococcus neoformans
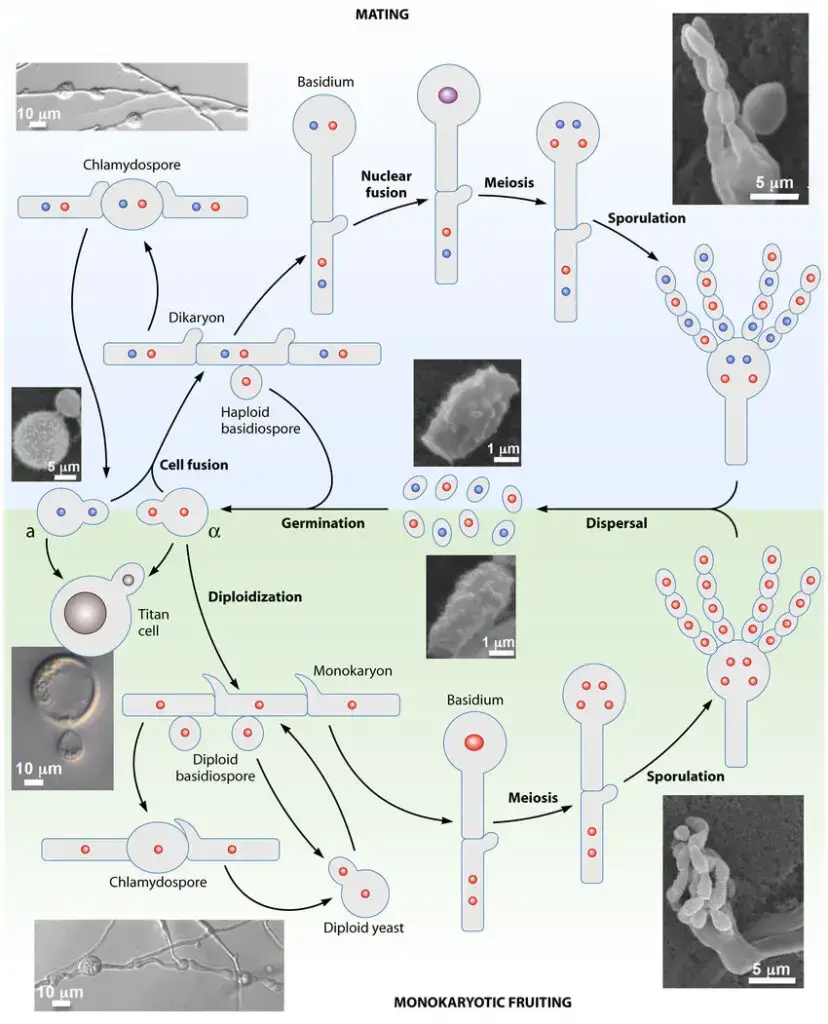
- C. neoformans exists as a budding yeast and is capable of metamorphosing into a filamentous form.
- C. neoformans’ morphogenesis is governed by two major differentiation pathways: mating and monokaryotic maturation.
- The mating pathway is initiated when haploid cells of opposite mating types fuse to form dikaryotic filaments.
- During hyphal development, blastospores may emerge from hyphae, and some hyphal cells may proliferate and form chlamydospores.
- Meiosis occurs as the basidium develops, resulting in the formation of four chains of basidiospores.
- In the absence of a mating partner, monokaryotic fruiting occurs when haploid spores produce filaments and basidiospores in response to acute nitrogen starvation or water deprivation.
- Meiosis occurs during the basidium development stage, and four chains of haploid basidiospores are produced.
- In a human host, C. neoformans can form exceptionally large polyploid cells known as “titan cells” or “giant cells.”
- Alongside their cartoon representations, microscopy images of the numerous stages of the C. neoformans life cycle are included.
- Red circles represent MATalpha nuclei, blue circles represent MATa nuclei, purple circles represent diploid nuclei with a/ content, and gray circles represent either MATalpha or MATa nuclei.
Virulence Factors of Cryptococcus neoformans
Cryptococcus neoformans is a fungal pathogen that causes life-threatening infections, primarily in immunocompromised individuals. Some of the virulence factors of Cryptococcus neoformans are:
- Capsule: The polysaccharide capsule surrounding the fungal cell is the major virulence factor of Cryptococcus neoformans. It interferes with phagocytosis, complement-mediated killing, and recognition by the host immune system.
- Melanin: Cryptococcus neoformans produces melanin, which has been shown to protect against host immune defenses, such as oxidative stress and phagocytosis.
- Phospholipase: Cryptococcus neoformans produces phospholipase enzymes that hydrolyze host cell membrane phospholipids, contributing to host cell damage and release of nutrients.
- Laccase: Laccase is an enzyme produced by Cryptococcus neoformans that is involved in melanin synthesis and also has antioxidant properties that help protect the fungus against host immune defenses.
- Urease: Cryptococcus neoformans produces urease, which contributes to fungal survival in the host by generating ammonia that helps neutralize acidic environments and reduce host immune responses.
- Mannitol: Cryptococcus neoformans produces mannitol, which may contribute to the osmotic balance of the fungus in the host and also act as a carbon source.
These virulence factors enable Cryptococcus neoformans to evade host immune defenses and establish infection.
Infection of Cryptococcus neoformans
- Cryptococcus neoformans is a pathogenic fungus that predominantly causes fatal infections in immunocompromised individuals, such as those with HIV/AIDS. The inhalation of yeast cells, which are desiccated and light enough to be easily aerosolized, initiates the infection.
- The fungus obtains access to the body through the respiratory system, causing a minimal, transient pulmonary infection. It is possible for the primary pulmonary infection to be asymptomatic or to mimic influenza, and it resolves spontaneously. In immunocompromised patients, the yeast replicates and travels to other organs, particularly the Central Nervous System (CNS).
- About 15% of HIV/AIDS patients are susceptible to Cryptococcus neoformans infection, which causes mild infections of the pulmonary system that extend to the skin, bones, viscera, and central nervous system.
- When the CNS is compromised, cryptococcal meningitis develops. Skin, adrenal glands, bone, the eye, and the prostate gland are also prevalent sites of dispersion.
- The polysaccharide capsule encircling the fungal cell is Cryptococcus neoformans’ primary virulence factor. It inhibits phagocytosis, complement-mediated killing, and host immune system recognition.
- Additionally, Cryptococcus neoformans generates melanin, which has been demonstrated to protect against host immune defenses such as oxidative stress and phagocytosis.
- The fungus also produces phospholipase enzymes that hydrolyze the phospholipids of host cell membranes, contributing to host cell degradation and nutrient release.
- Cryptococcus neoformans generates urease, which contributes to fungal survival in the host by producing ammonia that neutralizes acidic environments and suppresses host immune responses.
- Cryptococcus neoformans also produces the laccase enzyme, which is involved in melanin synthesis and has antioxidant properties that protect it from host immune defenses.
- In addition, the fungus produces mannitol, which may contribute to the osmotic balance of the fungus within its host as well as serve as a carbon source. These virulence factors allow Cryptococcus neoformans to circumvent the host’s immune defenses and establish an infection.
- Patients receiving Highly Active Antiretroviral Therapy (HAART) and cryptococcal meningitis are likely to develop immune reconstitution inflammatory syndrome (IRIS), which significantly exacerbates the illness. This is also prevalent in Tuberculosis patients with HIV.
- There is a minimal granulomatous reaction that causes inflammation. Infection with Cryptococcus neoformans is a severe condition requiring prompt diagnosis and treatment, particularly in immunocompromised individuals. Serious complications, such as meningitis and disseminated disease, can be avoided through early diagnosis and treatment.
Clinical features of Cryptococcus neoformans infection
- Cryptococcosis is a fungal infection induced by C. neoformans that is commonly associated with immunocompromised individuals, such as AIDS patients.
- Common symptoms of cryptococcosis include flu-like symptoms, chronic meningitis resembling a brain tumor, fungal meningitis, and diseases mimicking tuberculosis or mycobacteria.
- In patients with cryptococcosis, meningitis symptoms include severe headaches, cervical stiffness, disorientation, and photophobia. In some cases, patients may also have lesions on their skin, lungs, and bones, which can contribute to muscle weakness and dystrophy that prevents them from moving.
- Cryptococcosis can be fatal if left untreated, particularly in immunocompromised patients. Up to 15% of HIV-positive patients develop cryptococcosis, which is a primary cause of morbidity and mortality in AIDS patients.
- In organ transplant recipients and patients receiving immunosuppressive therapy for cancer or autoimmune diseases, it is also a leading cause of death.
- In addition to AIDS patients, those with sarcoidosis, diabetes mellitus, chronic renal failure, and chronic obstructive pulmonary disease (COPD) are at risk for developing cryptococcosis. Chemotherapy and the use of immunosuppressive medications are additional risk factors.
- Cryptococcosis must be diagnosed and treated promptly to prevent its progression and reduce the risk of complications.
- Amphotericin B and fluconazole are the most commonly prescribed antifungal medications for the treatment of cryptococcosis. In severe cases, surgery may be required to remove fungal masses or relieve cerebral pressure.
- Patients with AIDS or other immune deficiencies are required to receive lifelong antifungal treatment to prevent disease relapse.
Laboratory Diagnosis Methods of Cryptococcus neoformans
Cryptococcus neoformans is a fungal pathogen that can cause severe infections, especially in immunocompromised individuals. Laboratory diagnosis of cryptococcosis involves several methods, including specimen preparation, staining and microscopic examination, cultural examination, biochemical characterization, and serological examination. Here’s a detailed description of each method:
- Specimen and specimen preparation: Specimens for diagnosis include cerebrospinal fluid, tissue, exudates, sputum, blood, cutaneous scrapings, and urine. Spinal fluid is centrifuged before microscopic examination and culture.
- Staining and microscopic examination: Several stains are used for the microscopic examination of Cryptococcus neoformans, including Gram staining, India ink staining, and mucicarmine staining. Gram staining can reveal round cells with Gram-positive granular inclusion on a pale lavender cytoplasmic background or Gram-negative lipoid bodies. Negative staining with India ink is a quick method for the identification of Cryptococcus neoformans, as the cell capsule surrounding the yeast cells creates a halo around the cells. Mucicarmine staining is specific for the identification of the polysaccharide cell walls of Cryptococcus neoformans in tissue samples.
- Cultural examination: Culture examination uses Sabouraud Dextrose Agar (SDA) and incubation at room temperature or 37°C. Colonies of Cryptococcus neoformans develop within a few days. Cycloheximide-containing media can inhibit Cryptococcus, and this should be avoided. Urease production can be detected in the culture media. On an appropriate diphenolic substrate, the phenoloxidase (or laccase) of C. neoformans produces melanin in the cell walls, and colonies develop a brown pigmentation.
- Biochemical characterization: Biochemical characterization involves demonstrating the production of urease and laccase or a specific pattern of carbohydrate assimilations immediately after the cultural examination.
- Serological Examination: Serological examination involves the detection of antigens or antibodies in the patient’s blood, spinal fluid, or urine. Detection of the capsular polysaccharide antigen of Cryptococcus is the most common diagnostic test, using enzyme immunoassays or agglutination of latex particles coated with antibodies to the polysaccharide antigen. This test is sensitive and specific for antigen detection in cases of cryptococcal meningitis. Antibodies produced against the polysaccharide capsule antigen can also be quantified, which is useful for monitoring the course of the disease.
Treatment of Cryptococcosis
- The treatment of cryptococcosis involves antifungal therapy, and the choice of treatment depends on the severity of the infection and the patient’s immune status. The recommended treatment for cryptococcal meningitis in HIV-positive patients includes an initial induction phase with amphotericin B (conventional or liposomal) plus flucytosine for 2 weeks, followed by consolidation therapy with fluconazole for at least 8 weeks. In patients with severe disease or treatment failure, a second induction phase with amphotericin B plus flucytosine may be necessary. For non-HIV-infected patients, the duration of induction therapy is usually 4 to 6 weeks.
- For less severe infections, such as pulmonary cryptococcosis or skin lesions, fluconazole alone may be sufficient. In patients with allergies or intolerances to azoles, amphotericin B monotherapy may be used, although its toxicity limits its use.
- In addition to antifungal therapy, it is important to manage any underlying conditions that may be contributing to the patient’s immune deficiency. This may include the use of antiretroviral therapy in HIV-positive patients or the treatment of other immunosuppressive conditions.
- In some cases, surgical intervention may be necessary to manage complications of cryptococcosis, such as hydrocephalus or abscess formation.
- It is important to monitor patients closely during treatment, with regular assessments of clinical response and laboratory tests to evaluate the efficacy and safety of therapy.
Prevention and Control
Preventing and controlling cryptococcosis involves both general and specific measures, depending on the risk factors and the individual’s health status. Here are some strategies that can help prevent and control the disease:
- General measures:
- Avoid exposure to environments that are heavily contaminated with bird droppings or soil.
- Avoid exposure to dust, especially in construction sites or other settings where there may be a risk of fungal spore inhalation.
- Use personal protective equipment, such as face masks or respirators, when working in areas with a high risk of fungal exposure.
- Practice good hygiene, such as washing hands frequently and avoiding sharing personal items, especially with someone who has a weakened immune system.
- Maintain a healthy lifestyle, including regular exercise and a balanced diet, to boost the immune system.
- Specific measures:
- Antifungal prophylaxis: For individuals at high risk of developing cryptococcosis, antifungal medications may be prescribed as a preventive measure.
- Treatment of underlying medical conditions: Individuals with HIV/AIDS or other immune system disorders should receive appropriate medical treatment to manage their underlying condition and reduce the risk of developing cryptococcosis.
- Early diagnosis and treatment: Prompt diagnosis and treatment of cryptococcosis can reduce the risk of complications and improve outcomes. Healthcare professionals should consider cryptococcosis as a possible diagnosis in individuals with a weakened immune system and symptoms consistent with the disease.
- Environmental controls: In healthcare facilities or other settings where there is a risk of fungal exposure, measures such as ventilation systems, air filters, and disinfection protocols can help reduce the risk of cryptococcosis transmission.
FAQ
What is Cryptococcus neoformans, and how is it transmitted?
Cryptococcus neoformans is a type of fungus that can cause serious infections in humans. It is primarily transmitted through inhalation of fungal spores present in the environment, particularly in soil and bird droppings.
What are the symptoms of Cryptococcus neoformans infections?
Symptoms of Cryptococcus neoformans infections can vary depending on the affected organ system, but may include fever, headache, neck stiffness, confusion, blurred vision, skin lesions, and respiratory symptoms.
How is Cryptococcus neoformans diagnosed?
Diagnosis of Cryptococcus neoformans infections typically involves a combination of laboratory tests, including microscopy, culture, serological testing, and molecular testing.
What is the treatment for Cryptococcus neoformans infections?
Treatment for Cryptococcus neoformans infections typically involves antifungal medications, such as amphotericin B and fluconazole. In severe cases, surgery may be necessary to remove infected tissue.
Can Cryptococcus neoformans infections be prevented?
Prevention of Cryptococcus neoformans infections involves avoiding exposure to the fungus, particularly in high-risk settings such as construction sites and areas with large bird populations. Individuals with weakened immune systems may also benefit from prophylactic antifungal medications.
Who is most at risk for Cryptococcus neoformans infections?
Individuals with weakened immune systems, particularly those with HIV/AIDS, are most at risk for Cryptococcus neoformans infections. Other risk factors include organ transplant recipients, individuals on immunosuppressive therapies, and those with certain underlying medical conditions.
Is Cryptococcus neoformans contagious?
Cryptococcus neoformans is not typically contagious and is not spread from person to person.
How long does it take to recover from a Cryptococcus neoformans infection?
The length of recovery from a Cryptococcus neoformans infection can vary depending on the severity of the infection and the individual’s underlying health status. Treatment may last for several months, and long-term follow-up may be necessary.
Can Cryptococcus neoformans infections recur?
Cryptococcus neoformans infections can recur, particularly in individuals with weakened immune systems. Long-term follow-up and monitoring may be necessary to detect and treat recurrent infections.
What can be done to reduce the risk of Cryptococcus neoformans infections in high-risk settings?
In high-risk settings such as construction sites and areas with large bird populations, measures such as wearing protective clothing and masks, practicing good hygiene, and avoiding high-risk areas may help to reduce the risk of Cryptococcus neoformans infections.
References
- Voelz, Kerstin. (2010). Macrophage – cryptococcus interactions during cryptococcosis.
- Wang, L., & Lin, X. (2011). Mechanisms of unisexual mating in Cryptococcus neoformans. Fungal Genetics and Biology, 48(7), 651–660. doi:10.1016/j.fgb.2011.02.001
- Medical Microbiology by Jawertz, Melnick, and Adelberg, 26th Edition
- Microbiology by Prescott, 5the Edition
- Shapiro, Rebecca & Robbins, Nicole & Cowen, Leah. (2011). Regulatory Circuitry Governing Fungal Development, Drug Resistance, and Disease. Microbiology and molecular biology reviews : MMBR. 75. 213-67. 10.1128/MMBR.00045-10.
- Mada PK, Jamil RT, Alam MU. Cryptococcus. [Updated 2022 Oct 25]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK431060/
- Srikanta D, Santiago-Tirado FH, Doering TL. Cryptococcus neoformans: historical curiosity to modern pathogen. Yeast. 2014 Feb;31(2):47-60. doi: 10.1002/yea.2997. Epub 2014 Jan 19. PMID: 24375706; PMCID: PMC3938112.
- https://www.cdc.gov/fungal/diseases/cryptococcosis-neoformans/index.html#:~:text=Cryptococcus%20neoformans%20is%20a%20fungus,never%20get%20sick%20from%20it.
- https://en.wikipedia.org/wiki/Cryptococcus_neoformans
- https://www.cdc.gov/fungal/diseases/cryptococcosis-neoformans/symptoms.html