| Kingdom: | Fungi |
| Division: | Ascomycota |
| Class: | Saccharomycetes |
| Order: | Saccharomycetales |
| Family: | Saccharomycetaceae |
| Genus: | Candida |
| Species: | C. tropicalis |
- Candida tropicalis is a yeast species belonging to the Candida genus. It is a prevalent pathogen in neutropenic hosts, in whom it can spread to peripheral organs via the bloodstream.
- Antifungal remedies for invasive diseases include amphotericin B, echinocandins, and extended-spectrum triazoles.
- In the history of fungi, the name of the genus Candida, which is derived from the family Debaryomycetaceae, is derived from the Latin word “candidus,” which means “glowing white” and also alludes to something that is smooth and shiny.
- Genus Candida referred to any asexual yeast lacking the following characteristics: acetic acid production, pigments of red, pink, or orange, arthroconidia, unipolar or bipolar budding, enteroblastic-basipetal budding, blastoconidia formation on sympodulae, buds formation on stalks, triangular cells, needle-shaped terminal conidia, and the ability to grow on inositol as a sole carbon source.
- Despite the fact that 200 species have been identified within this genus, the taxonomy remains undefined and imprecise for a variety of reasons, including the reclassification of identified old species and the discovery of new species.
- This genus no longer contains taxa with positive diazonium blue B (DBB) tests. The extinct genera Oidium and Monilia were substituted for Candida.
- There are other taxa within the genus Candida that are comparable to Candida tropicalis. C. tropicalis and C. albicans share many pathogenic characteristics, whereas C. maltosa and C. sake are physiologically similar to C. tropicalis but can be distinguished by their growth at 35 °C (only C. sake shows a negative growth) and assimilation of soluble starch (only C. tropicalis assimilates soluble starch).
Habitat of Candida tropicalis
Candida tropicalis is a yeast species that inhabits a variety of natural environments and the human body. Here are some typical environments where Candida tropicalis is found:
- Human microbiota: Candida tropicalis is a normal component of the human microbiota, especially in the gastrointestinal tract, buccal cavity, and urogenital region. Under typical conditions, it can exist as a commensal organism without causing infection.
- Soil and vegetation: Candida tropicalis has been isolated from sediment and vegetation, indicating its presence in the environment. It can colonise plant surfaces and is present in the rhizosphere (the soil surrounding plant roots).
- Water sources: Candida tropicalis has been detected in rivers, lakes, and water reservoirs, among other water sources. It can endure and thrive in aquatic environments.
- Hospital settings: In clinical settings, Candida tropicalis is regarded as a significant opportunistic pathogen. It is able to colonise surfaces and medical devices, such as catheters and prosthetics, causing nosocomial infections.
- Animal hosts: Candida tropicalis has been isolated from mammals, birds, and reptiles, among other animal taxa. It may be found in the gastrointestinal tract and other animal body locations.
Morphological features of Candida tropicalis
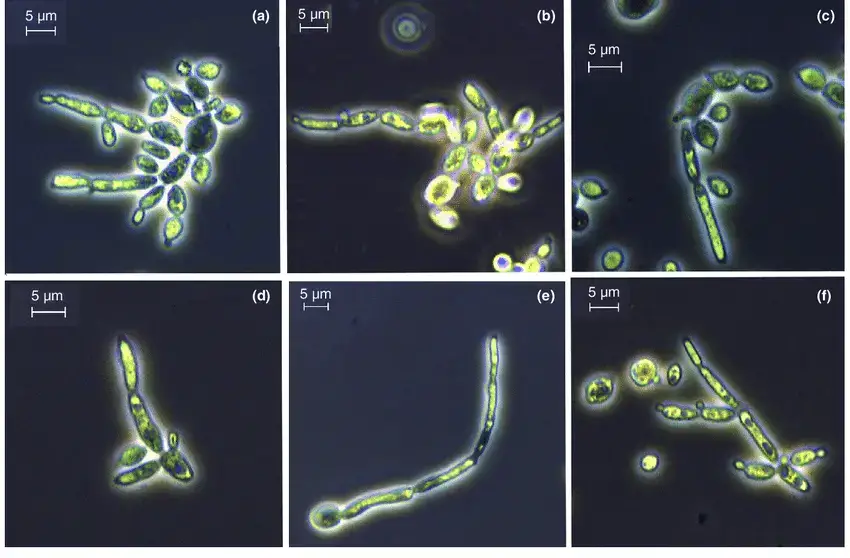
Candida tropicalis is a yeast species with distinct morphological characteristics. Here are the characteristic morphological features of Candida tropicalis:
- Cellular shape: Candida tropicalis cells are ovoid to elongated in shape, resembling yeast that is growing. The diameter of the cells can range from 3 to 8 micrometres on average.
- Budding pattern: Candida tropicalis reproduces via budding, a process in which a tiny daughter cell emerges from a larger mother cell. Different locations on the mother cell are capable of producing cells with a single or multiple branches.
- Pseudohyphae formation: Under certain conditions, Candida tropicalis is capable of producing pseudohyphae. Pseudohyphae are chains of connected, elongated yeast cells. However, Candida tropicalis typically does not produce genuine hyphae, which are long, branching filaments.
- Cell wall structure: The cell wall of Candida tropicalis is made up of a variety of components, including Chitin and glucan. These components contribute to the yeast cell’s structure, rigidity, and interactions.
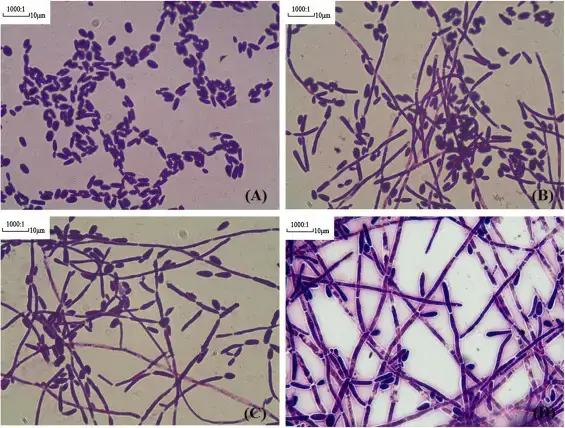
Cultural Characteristics of Candida tropicalis
Candida tropicalis exhibits specific cultural characteristics that can aid in its identification and differentiation from other Candida species. Here are the typical cultural features, the required medium for growth, and the optimal growth conditions for Candida tropicalis:
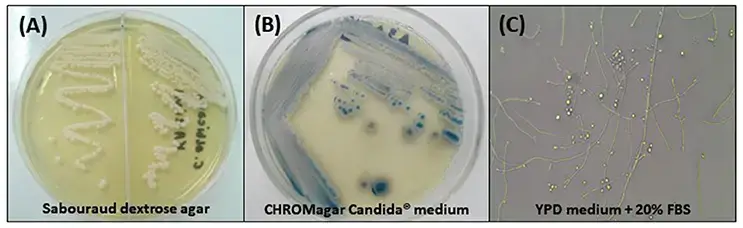

- Cultural characteristics:
- Colony morphology: Candida tropicalis colonies on solid agar media appear smooth, creamy, and usually white or cream-colored. However, colony color can vary depending on the specific strain or growth conditions.
- Texture: The colonies are typically smooth and have a moist or creamy texture.
- Growth rate: Candida tropicalis generally exhibits a moderate growth rate, which means it forms colonies at a moderate pace compared to other Candida species.
- Size: The colony size can range from small to moderate, typically measuring a few millimeters in diameter.
- Required medium for growth: Candida tropicalis can grow on various standard laboratory media used for cultivating yeasts and fungi, such as:
- Sabouraud dextrose agar (SDA): This medium contains dextrose, peptone, and agar, providing essential nutrients for yeast growth.
- Yeast extract peptone dextrose agar (YPD): YPD agar is a rich medium that supports the growth of Candida tropicalis and other yeasts. It contains yeast extract, peptone, dextrose, and agar.
- Optimal growth conditions: Candida tropicalis has the following preferred growth conditions:
- Temperature: The optimal temperature for growth is typically around 30-37°C (86-98.6°F), which corresponds to the normal human body temperature.
- pH: Candida tropicalis thrives in a slightly acidic to neutral pH range of approximately 5.0-7.0.
- Oxygen requirements: Candida tropicalis is a facultative anaerobe, meaning it can grow both in the presence and absence of oxygen. However, it generally prefers aerobic or microaerophilic conditions.
- Nutrient availability: Candida tropicalis requires a source of carbon, such as glucose or other carbohydrates, for energy and growth. It can utilize a variety of carbon sources for its metabolic needs.
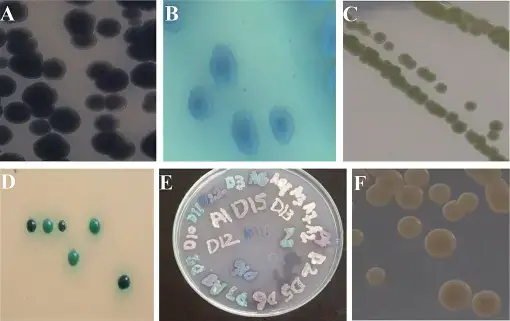
Pathogenesis of Candida tropicalis
Candida tropicalis is an opportunistic fungal pathogen capable of causing a variety of human infections. Its ability to colonise and invade host tissues, elude host immune defences, and produce virulence factors all play a role in the pathogenesis of Candida tropicalis. Key aspects of the pathogenesis of Candida tropicalis are as follows:
- Adherence and colonization: Candida tropicalis adheres to and colonises host surfaces, such as mucosal membranes or implanted medical devices, using adhesins present in its cell wall. This enables it to colonise and form biofilms, which are structured communities of fungal cells embedded within a matrix of extracellular material.
- Invasion and tissue damage: Candida tropicalis can invade host tissues by breaching epithelial barriers or by obtaining entry through damaged mucosal surfaces. It possesses enzymes, such as aspartyl proteases, that degrade host tissues, thereby facilitating tissue invasion and destruction.
- Immune evasion: Candida tropicalis possesses mechanisms for evading host immune responses. It is capable of modulating host immune cells, impairing their function and diminishing the efficacy of immune defences. It is also capable of producing factors that inhibit complement activation and phagocytosis, allowing it to evade host immune surveillance.
- Biofilm formation: Candida tropicalis is capable of biofilm formation on both biotic and abiotic surfaces. Biofilms provide protection against antifungal agents and the immune defences of the organism, making it more difficult to treat infections. In addition, these factors contribute to the persistence of Candida tropicalis infections and the emergence of drug resistance.
- Virulence factors: Candida tropicalis generates numerous virulence factors, which contribute to its pathogenesis. These include enzymes involved in tissue invasion and nutrient acquisition (e.g., proteases, lipases) as well as factors involved in stress response and immune modulation.
- Host variables: The susceptibility to Candida tropicalis infections can be increased by host-related factors such as latent immunodeficiency, compromised mucosal barriers, and the presence of indwelling medical devices. Certain conditions, such as diabetes or immunosuppressive treatments, can increase the risk of infection.
Transmission of Candida tropicalis
Candida tropicalis is transmitted predominantly through person-to-person contact, especially in healthcare settings. Here are the primary modes of Candida tropicalis transmission:
- Direct contact: Direct contact with an infected person is the most prevalent mode of transmission. This can occur when an individual comes into contact with the epidermis, mucous membranes, or bodily fluids (e.g., saliva, urine) of a person carrying Candida tropicalis.
- Healthcare-associated transmission: Candida tropicalis is transmissible in healthcare settings, including hospitals and long-term care facilities. It can spread via contaminated medical devices (e.g., catheters, ventilators), contaminated healthcare worker hands, and contaminated environmental surfaces.
- Indirect contact: Candida tropicalis can be transmitted indirectly through contact with contaminated objects or surfaces. Transmission can occur, for instance, by handling contaminated surfaces, medical equipment, or personal items that have been in contact with an infected individual.
- Vertical transmission: In rare instances, Candida tropicalis can be transmitted vertically from an infected mother to her newborn infant during childbirth. This can happen if the mother has a vaginal infection with Candida tropicalis at the time of delivery.
Virulence Factors of Candida tropicalis
Candida tropicalis, like other pathogenic Candida species, possesses multiple virulence factors that contribute to its ability to cause infections and elude the immune system of the host. The following are some of the most important virulence factors associated with Candida tropicalis:
- Adhesins: Candida tropicalis generates adhesins, such as Als proteins, Hwp1, and Epa proteins, on its cell surface, which facilitate its adhesion to host cells and surfaces. Adherence is a crucial initial step in colonisation and infection establishment.
- Biofilm formation: Candida tropicalis is capable of producing biofilms, which are structured communities of fungal cells encased in a protective extracellular matrix. Biofilms increase Candida tropicalis’ resistance to host immune responses and antifungal agents, making it more challenging to treat infections.
- Secreted proteases: Candida tropicalis secretes aspartyl proteases (Saps), such as Sap2 and Sap6, which are capable of degrading host tissues and extracellular matrix components. These proteases contribute to tissue invasion, immune evasion, and nutrient acquisition.
- Phospholipases: Candida tropicalis produces phospholipases, including phospholipase B (Plb1 and Plb2), which can hydrolyze phospholipids found in the membranes of host cells. This process facilitates the invasion and destruction of tissue.
- Hemolysins: Candida tropicalis is capable of producing hemolysins such as Candidalysin, which disrupt host cell membranes and result in red blood cell lysis. Hemolysins contribute to tissue injury and activation of the immune system.
- Mechanisms of antifungal resistance: Candida tropicalis has a greater propensity for antifungal resistance than other Candida species. It can acquire resistance to commonly employed antifungal agents, such as fluconazole and echinocandins, via multiple mechanisms, including mutations in drug target genes and efflux pump overexpression.
- Immune modulation: Candida tropicalis possesses mechanisms to modulate host immune responses. It can evade or suppress host immune defences by interfering with complement activation, phagocytosis, and cytokine signalling pathways.
- Toxins: C. tropicalis generates several toxins, including proteases, lipases, and phospholipases. These toxins can wreak havoc on the cells and tissues of the organism and aid the fungus in evading the immune system.
- Capsule: C. tropicalis cells can produce a capsule. This aids the fungus in evading the immune system.
- Surface proteins: C. tropicalis cells express a variety of surface proteins that are capable of interacting with host cells and tissues. These proteins aid fungi in adhering to host cells, invading host tissues, and evading the immune system.
- Phenotypic switching: C. tropicalis cells are capable of transitioning between distinct phenotypes. Thus, the fungus is able to adapt to various environmental conditions and evade the immune system.
Laboratory Diagnosis of Candida tropicalis
Typically, the laboratory diagnosis of Candida tropicalis infections entails a combination of direct microscopic examination, culture techniques, and identification techniques. Here are the most common laboratory methods for diagnosing Candida tropicalis:
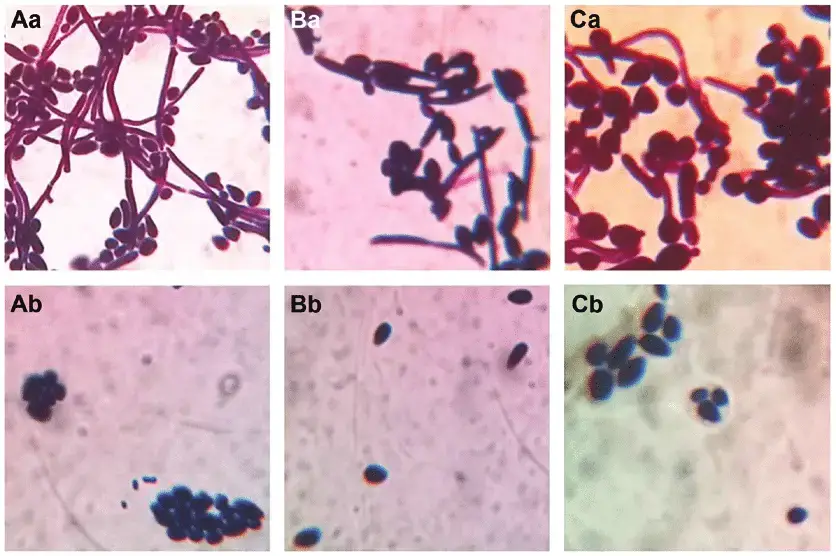
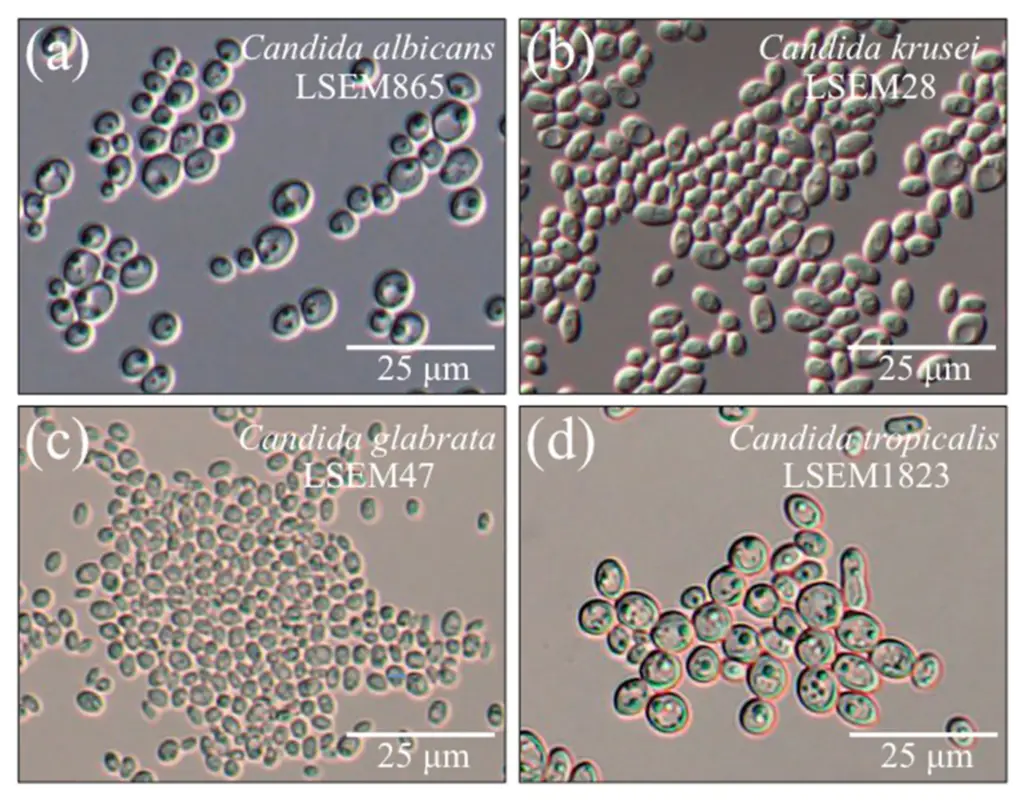
- Microscopic examination: Direct microscopic examination of clinical specimens such as vaginal swabs, oral swabs, and skin scrapings is possible using techniques such as potassium hydroxide (KOH) moist mount and Gramme staining. Candida tropicalis manifests as yeast cells with or without pseudohyphae, which aids in initial identification.
- Culture techniques: Candida tropicalis can be cultured on a variety of fungal media, including Sabouraud dextrose agar (SDA) and chromogenic agar, which facilitates its isolation and identification. The specimens are inoculated onto agar dishes and incubated for several days at the appropriate temperature (usually 30-37°C). On solid media, Candida tropicalis colonies typically appear as creamy or white colonies.
- Germ tube test: The germ tube test is a biochemical procedure used to distinguish Candida tropicalis from other Candida species. Yeast cells are incubated in human or animal serum at 37°C for two to three hours. Candida tropicalis lacks genuine germ tubes, unlike other Candida species such as Candida albicans.
- Chromogenic agar: Chromogenic agar is a culture medium that can assist in the identification of Candida species. It contains particular substrates that induce colour reactions when metabolised by various Candida species. On chromogenic agar, Candida tropicalis typically produces a distinct colour reaction, facilitating its identification.
- Biochemical tests: To confirm the identification of Candida tropicalis, additional biochemical assays, such as carbohydrate assimilation tests (e.g., API 20C AUX system), can be performed. These tests evaluate the yeast’s capacity to metabolise various carbon sources and can be used to distinguish Candida tropicalis from other Candida species.
- Molecular methods: For the swift and accurate identification of Candida tropicalis, molecular techniques such as polymerase chain reaction (PCR) and DNA sequencing can be used. These methods target particular genetic regions or genes that are exclusive to Candida tropicalis and can provide a conclusive identification.
Clinical Features of Candida tropicalis infection
Candida tropicalis is an opportunistic fungal pathogen that can cause a range of infections in humans. The clinical features of Candida tropicalis infection can vary depending on the site of infection, the underlying host factors, and the immune status of the individual. Here are some common clinical features associated with Candida tropicalis infections:
- Mucocutaneous infections:
- Oropharyngeal candidiasis (thrush): Candida tropicalis can cause white or creamy patches on the tongue, inner cheeks, or other areas of the oral cavity. These patches may be accompanied by pain, difficulty swallowing, or altered taste sensation.
- Vulvovaginal candidiasis: Candida tropicalis can cause itching, burning, redness, and a thick, white vaginal discharge in women. Vulvar irritation and discomfort may also be present.
- Invasive candidiasis:
- Bloodstream infections (candidemia): Candida tropicalis can enter the bloodstream, leading to candidemia. Symptoms may include fever, chills, hypotension, and organ dysfunction. Candidemia can be severe and potentially life-threatening, particularly in immunocompromised individuals.
- Intra-abdominal infections: Candida tropicalis can cause intra-abdominal infections, such as peritonitis or abscesses, especially in individuals with underlying gastrointestinal disorders or surgical interventions.
- Urinary tract infections: Candida tropicalis can cause urinary tract infections, particularly in patients with indwelling urinary catheters or underlying urinary tract abnormalities. Symptoms may include dysuria, urinary frequency, urgency, or flank pain.
- Systemic infections: In immunocompromised individuals, Candida tropicalis can disseminate to various organs, leading to deep-seated systemic infections, such as endocarditis, osteomyelitis, or meningitis. These infections are usually associated with high morbidity and mortality rates.
Treatment of Candida tropicalis infections
The treatment of Candida tropicalis infections typically involves antifungal therapy, which aims to eliminate the fungal infection and alleviate associated symptoms. The choice of antifungal agents and treatment duration may depend on the site and severity of the infection, the immune status of the patient, and any underlying conditions. Here are the commonly used treatment approaches for Candida tropicalis infections:
- Antifungal medications:
- Azole antifungals: Azole drugs such as fluconazole, voriconazole, or isavuconazole are often considered as the first-line treatment for Candida tropicalis infections, especially for less severe infections. They work by inhibiting the synthesis of ergosterol, a key component of fungal cell membranes.
- Echinocandins: Echinocandin drugs, such as caspofungin, micafungin, or anidulafungin, are usually reserved for severe or invasive Candida tropicalis infections. Echinocandins target the fungal cell wall by inhibiting the synthesis of β-(1,3)-D-glucan, resulting in cell wall disruption and fungal cell death.
- Polyenes: Although less commonly used, polyene antifungals like amphotericin B may be considered for severe or refractory Candida tropicalis infections. They bind to fungal cell membranes, causing membrane permeability and subsequent cell death.
- Combination therapy: In certain cases, combination therapy with two or more antifungal agents may be employed, especially for severe or recurrent infections, or in cases of antifungal resistance. Combination therapy aims to enhance the efficacy and broaden the spectrum of antifungal coverage.
- Antifungal susceptibility testing: It is important to perform antifungal susceptibility testing to determine the sensitivity of Candida tropicalis isolates to different antifungal drugs. This helps guide the selection of appropriate antifungal agents and optimize treatment outcomes.
- Management of underlying conditions: Treating underlying conditions, such as immunosuppression, diabetes mellitus, or the removal of infected medical devices (e.g., catheters), is essential for successful management of Candida tropicalis infections. Addressing predisposing factors can help prevent recurrence or further complications.
Candida tropicalis Antifungal resistance
Candida tropicalis has exhibited a greater antifungal resistance propensity than other Candida species. Antifungal resistance refers to the fungus’ ability to survive and grow in the presence of antifungal drug concentrations that would typically inhibit or kill susceptible strains. Here are a few essential details about antifungal resistance in Candida tropicalis:
- Fluconazole resistance: Candida tropicalis exhibits greater resistance to fluconazole, a widely used azole antifungal, than other Candida species. This resistance is frequently associated with specific genetic mutations in the lanosterol 14-demethylase (ERG11 gene) target enzyme of fluconazole or with the upregulation of efflux pumps that can actively remove the drug from the fungal cell.
- Echinocandin resistance: While Candida tropicalis is generally more susceptible to echinocandins than other Candida species, there have been reports of echinocandin resistance in Candida tropicalis clinical isolates. Echinocandin resistance mechanisms in Candida tropicalis include mutations in the FKS1 and FKS2 genes, which code for -(1,3)-D-glucan synthase, the target enzyme of echinocandins.
- Multidrug resistance: Candida tropicalis isolates resistant to multiple classes of antifungal medications, such as azoles, echinocandins, and polyenes, have been reported. Multidrug resistance can substantially restrict available treatment options and complicate the management of Candida tropicalis infections.
- Clinical implications: In immunocompromised or critically ill patients, antifungal resistance in Candida tropicalis can result in treatment failures, persistent infections, and an increase in mortality rates. Effective management requires accurate antifungal susceptibility testing and individualised treatment strategies.
- Risk factors: Prior exposure to antifungal agents, protracted or repeated antifungal therapy, the presence of implanted medical devices, and healthcare-associated infections all contribute to the development of antifungal resistance in Candida tropicalis. In addition, genetic variability and the possibility of horizontal gene transfer between Candida species contribute to the emergence and spread of antifungal resistance.
- Surveillance and research: Continuous surveillance of antifungal resistance patterns is essential for monitoring trends and informing treatment recommendations. It is necessary to conduct additional research into the mechanisms of antifungal resistance in Candida tropicalis in order to enhance our understanding of resistance emergence and devise new treatment strategies.
Prevention and Control of Candida tropicalis
Candida tropicalis prevention and control focuses on reducing the risk of infection and limiting its transmission. Here are some important strategies for the prevention and control of Candida tropicalis:
- Infection control practices in healthcare settings: To prevent the spread of Candida tropicalis in healthcare facilities, strict adherence to infection control protocols is required. This involves proper hand hygiene, the proper use and management of medical devices, the implementation of contact precautions, and routine cleaning and disinfection of patient care areas.
- Catheter care: Catheter care is essential, as indwelling urinary or intravascular catheters can function as potential sites of Candida tropicalis colonisation and infection. Infections can be prevented by adhering to sterile catheter insertion procedures, maintaining the catheter site routinely, and removing catheters as soon as they are no longer required.
- Antifungal stewardship: Important for preventing the emergence of antifungal resistance is the prudent and appropriate use of antifungal agents. Based on clinical evidence and susceptibility testing, antifungal stewardship programmes encourage the prudent use of antifungals, including the selection of the appropriate drug, optimal dosing, and duration of therapy.
- Environmental hygiene: Effective cleansing and disinfection of environmental surfaces, especially in healthcare settings, can assist in eradicating Candida tropicalis and lowering the risk of transmission. The standard cleaning protocol should include the use of proved antifungal disinfectants.
- Patient education: Patient education is crucial for preventing Candida tropicalis infections. Patients can be empowered to reduce their risk of infection by receiving information on good hygiene practises, such as handwashing, appropriate perineal care, and oral hygiene.
- Immunocompromised patients: Immunocompromised patients require special attention because they are more susceptible to Candida tropicalis infections. In this population, strategies to reduce the risk of infection include optimising their underlying condition, instituting stringent infection control measures, and considering antifungal prophylaxis in high-risk situations.
- Surveillance and monitoring: Regular surveillance of Candida species in healthcare settings can help identify trends in resistance patterns, assess the efficacy of infection control measures, and direct treatment. Close observation of high-risk patients, such as those in intensive care units or undergoing invasive procedures, can aid in the early detection and prompt treatment of Candida tropicalis infections.
FAQ
What is Candida tropicalis?
Candida tropicalis is a species of yeast that can cause infections in humans, particularly in immunocompromised individuals or those with underlying health conditions.
What types of infections can Candida tropicalis cause?
Candida tropicalis can cause a variety of infections, including bloodstream infections (candidemia), urinary tract infections, mucocutaneous infections (such as thrush and vulvovaginal candidiasis), and deep-seated systemic infections.
How is Candida tropicalis different from other Candida species?
Candida tropicalis is known for its higher rates of antifungal resistance compared to other Candida species, such as Candida albicans. It can also cause infections in different anatomical sites and may exhibit distinct virulence factors.
What are the risk factors for Candida tropicalis infections?
Risk factors for Candida tropicalis infections include immunosuppression, prolonged hospital stays, invasive medical procedures, indwelling medical devices (such as urinary catheters), previous exposure to antifungal agents, and underlying health conditions such as diabetes or malignancies.
How are Candida tropicalis infections diagnosed?
Candida tropicalis infections are diagnosed through a combination of clinical evaluation, microscopy, culture techniques, and sometimes molecular methods. Direct examination, culture on appropriate media, and identification tests are used to confirm the presence of Candida tropicalis.
What are the treatment options for Candida tropicalis infections?
Antifungal therapy is the mainstay of treatment for Candida tropicalis infections. Azole antifungals, echinocandins, and occasionally polyenes are commonly used. The choice of antifungal agent depends on factors such as the site and severity of infection, antifungal susceptibility, and patient factors.
Are there any natural remedies or preventive measures for Candida tropicalis infections?
While some natural remedies or dietary changes may help support overall health and immune function, there is no conclusive evidence that they can treat or prevent Candida tropicalis infections. Prevention primarily involves good hygiene practices, infection control measures, and addressing underlying risk factors.
Can Candida tropicalis infections be transmitted from person to person?
Candida tropicalis is considered an opportunistic pathogen, primarily infecting individuals with predisposing factors. It is not typically transmitted from person to person like a contagious infection, but rather originates from the individual’s own endogenous flora or from environmental sources.
Can Candida tropicalis infections be prevented?
While it may not be possible to completely prevent Candida tropicalis infections, certain measures can reduce the risk. These include practicing good hand hygiene, using proper catheter care, implementing infection control protocols, and optimizing the management of underlying conditions.
Are Candida tropicalis infections serious?
Candida tropicalis infections can range from mild to severe, depending on various factors. In immunocompromised or critically ill individuals, these infections can be more severe and may lead to complications. Prompt diagnosis and appropriate treatment are important to ensure favorable outcomes.
References
- Papon N, Courdavault V, Clastre M, Bennett RJ. Emerging and emerged pathogenic Candida species: beyond the Candida albicans paradigm. PLoS Pathog. 2013;9(9):e1003550. doi: 10.1371/journal.ppat.1003550
- Lockhart SR, Etienne KA, Vallabhaneni S, et al. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin Infect Dis. 2017;64(2):134-140. doi: 10.1093/cid/ciw691
- Posteraro B, Sanguinetti M. The evolution of antifungal resistance in Candida tropicalis. Drug Resist Updat. 2014;17(1-2):12-19. doi: 10.1016/j.drup.2014.03.002
- Guinea J. Global trends in the distribution of Candida species causing candidemia. Clin Microbiol Infect. 2014;20 Suppl 6:5-10. doi: 10.1111/1469-0691.12539
- Tsai MJ, Wu CY, Lin PC, et al. Characteristics of Candida bloodstream infections in tertiary care hospitals in Taiwan, 2000-2009: a retrospective multicenter study. PLoS One. 2014;9(4):e94817. doi: 10.1371/journal.pone.0094817
- Shin JH, Bougnoux ME, d’Enfert C, Kim SH, Moon CJ, Joo MY, et al. Cryptic diversity of the Candida parapsilosis complex includes a novel species, Candida orthopsilosis sp. nov., and its Och1p silent variant. FEMS Yeast Res. 2011;11(4): 465-74. doi: 10.1111/j.1567-1364.2011.00730.x
- Horn DL, Neofytos D, Anaissie EJ, et al. Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clin Infect Dis. 2009;48(12):1695-1703. doi: 10.1086/599039
- Moran C, Grussemeyer CA, Spalding JR, Benjamin DK Jr, Reed SD. Comparison of costs, length of stay, and mortality associated with Candida glabrata and Candida albicans bloodstream infections. Am J Infect Control. 2010;38(1):78-80. doi: 10.1016/j.ajic.2009.04.290
- Nagashima M, Yamamoto M, Matsumura M, et al. Candida tropicalis in haematological malignancy: a retrospective clinical study. Mycoses. 2019;62(2):140-146. doi: 10.1111/myc.12859
- Lohse MB, Gulati M, Johnson AD, Nobile CJ. Development and regulation of single- and multi-species Candida albicans biofilms. Nat Rev Microbiol. 2018;16(1):19-31. doi: 10.1038/nrmicro.2017.107
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.