Although Aspergillus flavus is widely distributed in soil and decaying plant material, it is most often associated with warm, humid agricultural environments where crops are produced.
Aspergillus flavus is classified as a filamentous fungus within the genus Aspergillus.
When stored grains (maize/peanuts,cottonseed) are exposed to moisture and heat, contamination is caused frequently by this fungus and aflatoxins are produced as toxic secondary metabolites. Spores, called conidia, are produced in abundance, and they are readily dispersed through air currents, dust and handling.
Because aflatoxin B₁ is regarded as a highly potent carcinogen, regulatory limits have been established in many countries, and food-safety programs are enforced to reduce exposure. Colony appearance is usually described as yellow-green to olive with a powdery surface due to heavy sporulation.
Although many isolates are toxigenic, considerable genetic variability is observed among strains and toxin-production capacity is strain-dependent. In clinical practice infections are reported, and invasive disease is documented mainly in immunocompromised patients. When conidia are inhaled by susceptible hosts, germination may be followed by tissue invasion which is facilitated by hyphal growth and secreted enzymes.
Researchers study its genetics and ecology to understand why some strains produce high levels of toxin. Control strategies are recommended for stored commodities (drying, aeration, good sanitation), and biological approaches such as use of non-toxigenic strains are promoted to outcompete toxigenic isolates.
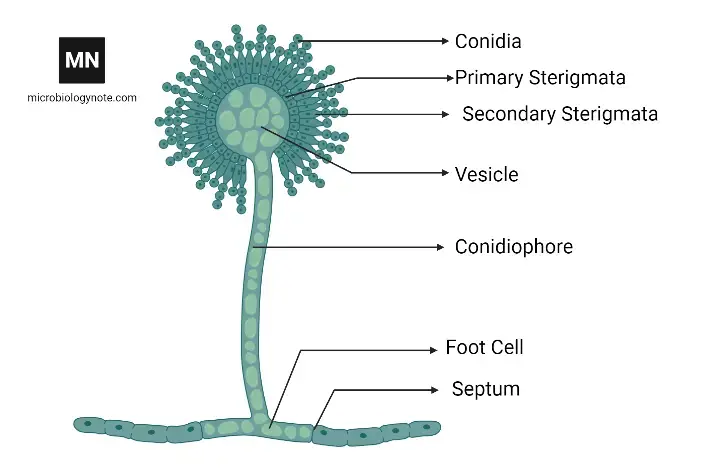
While culture and microscopy can be used for detection, molecular identification methods are increasingly preferred, and aflatoxin residues are quantified to confirm contamination. The fungal cell wall is composed mainly of chitin, glucans and various proteins, and the conidiophores are characterized by a vesicle that bears chains of conidia.
Patients often experience allergic symptoms; keratitis or sinusitis can result from infection by this organism. Environmental persistence is supported by the ability of conidia to remain viable for long periods, thereby increasing the likelihood of airborne exposure and recontamination of crops.
It survives in soil and on crop residues, especially when moisture and temperature conditions are favorable. Epidemiological associations have been shown between chronic aflatoxin exposure and hepatocellular carcinoma, and dose-response relationships are being evaluated to set safe limits.
Diagnostic confusion is created because morphology overlaps with other Aspergillus species, hence sequence-based methods are relied upon for species-level identification. Economic losses are caused by crop spoilage and trade restrictions when contamination is detected.
Although complete elimination is unrealistic, public-health interventions and improved agricultural practices are being promoted to mitigate risks, and consumer education is encouraged to reduce exposure to contaminated food.
Habitat of Aspergillus flavus
- It is widely accepted that Aspergillus flavus is ubiquitously distributed in soil environments, where it persists as conidia or sclerotia.
- In many agricultural fields, organic debris and crop residues are colonized by A. flavus and serve as a reservoir for future growth.
- Within stored grains, legumes, tree nuts and seeds, contamination is frequently observed and the fungus is sustained under favorable moisture/temperature conditions.
- In indoor settings (e.g., damp buildings, storage rooms, basements), growth is often allowed when moisture accumulates and ventilation is poor.
- Because hot and humid climates are favorable, tropical and subtropical zones are often more heavily inhabited; semi-arid regions also host this fungus under stress conditions.
- Within plant tissues (especially in stressed or injured parts) colonization is sometimes initiated and latent infection is maintained until harvest.
- In soils under harsh conditions, sclerotia are formed and are tolerated long periods of dormancy until favorable conditions return.
- In many cases, the fungus is considered thermotolerant and is able to grow at relatively high temperatures (e.g. ~37 °C or above) which broadens its habitat range.
- Under storage conditions of high humidity or moisture, proliferation is encouraged and aflatoxin production is triggered in susceptible substrates.
- During drought stress of plants, colonization is easier and the fungus is more competitive over other microbes.
- On dead plant matter or decaying vegetative tissues in fields or compost piles, saprophytic growth is supported.
- Between 12 °C and up to ~48 °C, growth may occur in different substrate types though the optimum is nearer to body-temperature ranges.
- In dust, air , and aerosols (especially in handling of contaminated materials), spores are dispersed from one habitat to another.
- In regions across ~25°–35° latitude (tropical/subtropical belts) prevalence is often higher, as noted for many Aspergillus species.
- In storage, if moisture is kept very low, the habitat becomes limiting and growth is suppressed (thus low water activity is used as a control).
Morphology of Aspergillus flavus
- The colonies are often yellow-green, olive or yellowish, and are initially whitish at growth front before conidia appear.
- A floccose / woolly or velvety texture is often produced on solid media, and radial grooves or sulcations are sometimes observed in older colonies.
- The reverse side of colony (underside) is commonly pale, cream or ochraceous in many isolates.
- Hyphae are septate, hyaline, and branching filaments constitute the vegetative mycelium.
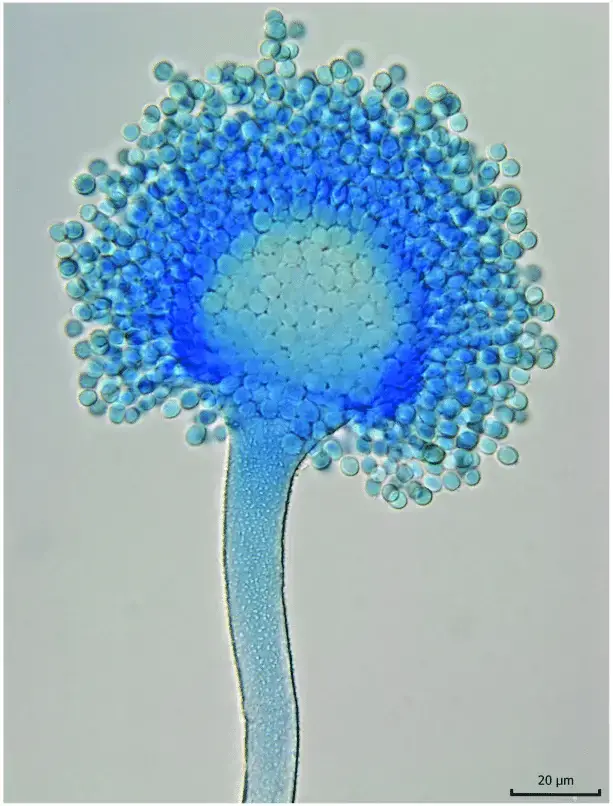
- Conidiophores are borne singly, are rough-walled (coarsely rugose), mostly hyaline and often uncolored until sporulation onset.
- In many cases the stipe of conidiophore attains lengths between ~400 and 800 μm, with roughened wall surface, and diameter varying with strain.
- The vesicle (terminal swelling) is globose to subglobose or somewhat clavate, measuring ~20–45 μm or in range ~23.7–44.3 μm, depending on isolate.
- Phialides and metulae arrangements are variable: many isolates are biseriate (metulae + phialides), though some isolates remain uniseriate (phialides directly on vesicle) in certain conditions.
- Conidial heads appear radiate, and with age become loosely columnar, sometimes splitting into loose columns (300–400 μm diameter) in dense sporulation.
- Conidia are generally subglobose, smooth (or slightly irregular), hyaline to greenish in mass, with diameters in the order of ~2.1–6.1 × 1.7–6.5 µm (depending on isolate) under various culture conditions.
- Sclerotia (compact masses of hardened mycelium) are produced by many isolates; two morphotypes exist — S strains (small sclerotia < 400 µm) and L strains (large sclerotia > 400 µm).
- The S morphotype usually produces many small sclerotia and tends to conidiate less abundantly, whereas L morphotype produces fewer but larger sclerotia and often yields abundant conidia.
- In many isolates the onset of sporulation transitions the colony from white mycelial growth to pigmented conidial mass, progressively covering the surface.
- Variation is seen among isolates in sporulation density, conidial size, vesicle shape/size, and texture (granular, floccose, smooth) that complicates morphological identification.
- Under microscopy, the mycelium often appears whitish / hyaline at colony margins, and conidiophores with phialides radiating from all sides of vesicle are evident.
- Some irregularities in morphology (slightly rough / ornamented conidial surfaces, variation in conidiophore wall texture) are observed across different strains or under stress conditions.
Hosts
Many agricultural crops are hosts of A. flavus, and these include cereal grains (e.g. maize/corn, sorghum, rice), legumes (e.g. peanuts, beans), and tree nuts (e.g. almonds, pistachios).
In seeds or kernels of these crops, the fungus may invade embryos / internal tissues, reducing germination and yield.
On plant surfaces (leaves, stems, silks), A. flavus may colonize wounded or stressed tissues, especially where insect damage or drought stress is present.
After harvest, stored grains / nuts / seeds become hosts because the fungus can grow under favorable moisture / temperature in storage.
In indoor environments (e.g. building materials, dust, ventilation ducts, damp walls, paper, textiles, wood) A. flavus has been isolated, hence non-plant hosts (substrates) also support its presence.
Mammals (including humans, cattle, horses, birds) are occasional hosts when A. flavus acts as an opportunistic pathogen, with infection of lungs, sinuses, eyes etc.
Insects and arthropods are sometimes implicated as hosts or vectors: A. flavus has been detected in insects and is considered to have a broad non-specialized host range across plants, insects, animals.
Fungi / decaying organic matter are not exactly “hosts” in disease sense, but A. flavus grows saprophytically on dead plant debris, soil organic matter and compost (i.e. non-living substrates) which act as reservoir “hosts.”
Aquatic or moist environments (water films on surfaces) occasionally support A. flavus growth on substrates (e.g. damp walls), making those surfaces incidental hosts / growth sites.
In human medical cases, paranasal sinuses, cornea (eye), lung tissue, skin wounds, bone (osteomyelitis) have been documented as sites where A. flavus can colonize / infect in susceptible hosts.
Cultural characteristics of Aspergillus flavus
- On potato dextrose agar at ~25 °C, Aspergillus flavus colonies are typically olive to lime-green, with a cream colored reverse side and rapid growth is observed.
- The colony surface texture is often described as woolly, cottony or somewhat granular, and radial grooves or sulcate patterns are sometimes produced.
- A dense felt of yellow-green conidiophores is often formed, and sporulation is usually abundant, covering much of colony surface over time.
- At early growth stages, colonies may appear whitish or pale before pigmentation sets in; later, greenish/olive tones dominate and white margins may persist until full sporulation.
- In many isolates, exudates (liquid droplets) are produced (clear to pale brown) and may become embedded in the mycelium.
- On Czapek agar / Czapek-Dox medium, colonies are granular, flat, often with radial grooves, starting yellow but rapidly darkening to bright / dark yellow-green.
- Some isolates produce sclerotia (dark brown or black) in older cultures, and these may be scattered or concentrated depending on morphotype.
- The growth rate is fast: colony diameters of ~3–5 cm are reached in ~7 days on Czapek agar at 25 °C in many strains.
- Poor growth is seen on certain selective media (e.g. creatinine sucrose agar, CREA), and colony reverse colors may vary (e.g. orange on AFPA medium) in reaction to medium constituents.
- Two morphotypic groups (S and L) are distinguished in culture: S morphotype strains tend to form many small sclerotia and often sporulate less, while L morphotype strains tend to produce abundant conidia and fewer large sclerotia.
- In many isolates, sporulation begins from the center, proceeds radially, and the white border (unsaturated region) is gradually overtaken by conidial pigmentation as growth continues.
- On media where intermediate reactions occur (e.g. AFPA), colony reverse coloration or pigment reactions (orange / golden) may be seen, and soluble pigment production is variably observed.
- Abundant sporulation often causes the colony to change from a woolly / white mycelial mass to dense, powdery green surface, and sometimes splitting of conidial heads into loose columns is seen in aging cultures.
- Reverse or underside (back side) of many colonies remains pale, cream or buff in many isolates, though variation exists depending on strain and medium.
Culture media used for growth of Aspergillus flavus
Here is a list of commonly used culture media for growth of Aspergillus flavus:
- Czapek-Dox Agar (CZA / Czapek’s medium) is often used as a synthetic medium with sucrose as carbon source and nitrate as nitrogen source, and growth of A. flavus is supported.
- Potato Dextrose Agar (PDA) is widely used (for general fungal cultivation) and A. flavus is readily grown on it under laboratory conditions.
- Aspergillus flavus and parasiticus Agar (AFPA) is a selective / differential medium in which A. flavus colonies develop yellow-orange coloration at the reverse side (due to reaction of ferric ions with aspergillic acid) which helps in distinguishing from others.
- Malt Extract Agar (MEA) is used frequently, especially in morphological / taxonomic studies, because good sporulation and colony features are induced.
- Oxytetracycline Glucose Yeast Extract Agar (OGY) is applied in some morphological comparative studies of A. flavus to enhance certain colony features
- Coconut Milk Agar (CMA) is used in research settings to provoke morphological / pigmentation traits (especially under UV or special lighting) for A. flavus.
- Yeast Extract Agar (YEA / YE medium) is also used, especially for growth and morphological observation, sometimes in combination with other media to contrast growth traits.
- For environmental sampling, Dichloran-glycerol agar (DG18) is used sometimes to restrict fast-growing molds and allow isolation of Aspergillus spp., including A. flavus, especially from indoor or environmental samples.
- Modified Rose Bengal based media (e.g. M-RB, modified rose bengal agar) have been used in studies to increase A. flavus isolation yield and suppress competitor fungi.
- In quality-control / food testing labs, selective / modified media including addition of antibiotics, fungistatic agents (e.g. dichloran) or inhibitors of bacterial growth are used so that A. flavus growth is facilitated while suppressing unwanted organisms.
- Sabouraud Dextrose Agar (SDA)[Sabouraud Dextrose Agar, also known as SDA, is a type of gel-like substance used to grow microorganisms like bacteria and fungi. It’s made up of a mixture of ingredients that provide food for the microorganisms, and it’s often used in laboratories to help scientists study and identify different types of microorganisms. Just like how people need food to grow and stay healthy, microorganisms need a good environment to grow and multiply, and SDA provides that environment. It’s kind of like a playground for microorganisms, where they can grow and play and be studied by scientists.] is commonly used for the isolation and cultivation of various fungi, including Aspergillus flavus. The colonies of Aspergillus flavus grown on SDA are white and soft, with a velvety texture that gradually turns yellowish-green due to the presence of a pigment in the conidial spores.
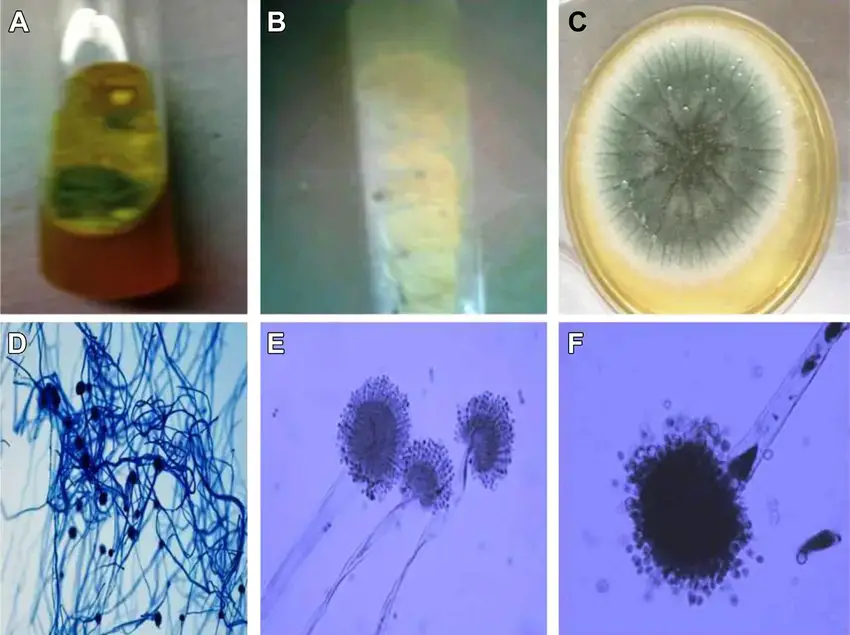
- Potato Dextrose Agar (PDA) is another commonly used agar for fungal isolation and cultivation. On PDA, the colonies of Aspergillus flavus show characteristic features such as green conidia and deep brown sclerotia mass.

- Malt extract agar (MEA) is a medium that supports the growth of a wide range of fungi, including Aspergillus flavus. The colonies of Aspergillus flavus grown on MEA are smooth and gradually change to an olive-green color. The fungus also produces colorless sclerotia on this medium.
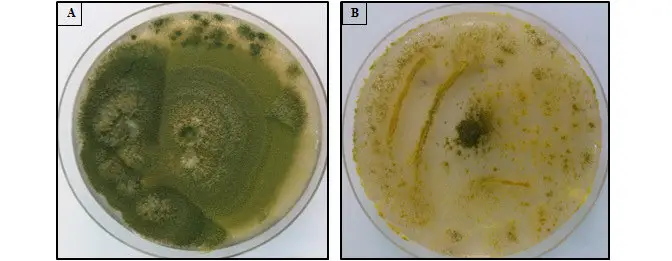
- Czapek yeast agar (CYA) is a selective medium used for the isolation and cultivation of Aspergillus species. The colonies of Aspergillus flavus on CYA appear after 7 days of incubation at either 25°C or 37°C, and they exhibit velutinous, grey-blue-green, and uniseriate conidial heads.
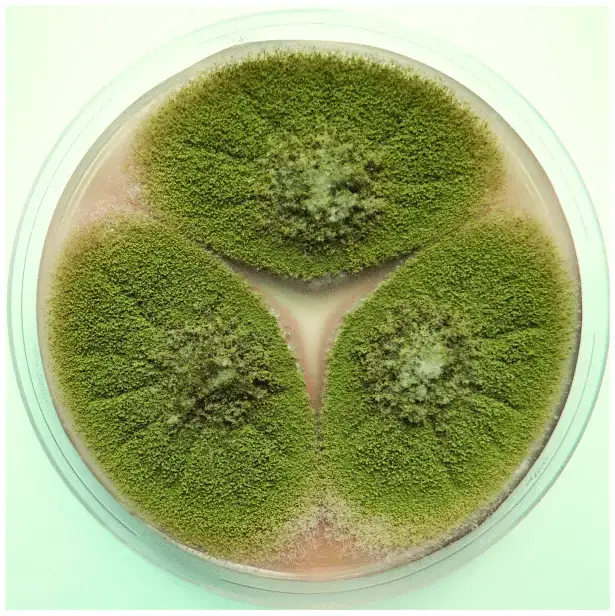
The life cycle of Aspergillus flavus
- In soil or decaying substrate, dormant sclerotia or mycelial fragments are maintained (survive) during unfavorable periods.
- When favorable conditions (moisture, temperature, nutrients) return, sclerotia germinate or mycelium expands and hyphae are formed.
- The hyphal growth is extended through substrate, branching and absorbing nutrients from surrounding organic matter.
- At appropriate stage, conidiophores are developed from aerial hyphae, and vesicles, phialides etc. are differentiated.
- Conidia (asexual spores) are produced in chains on phialides, and these spores are released into environment.
- The released conidia are dispersed by wind, insects, dust, or mechanical disturbance to new substrates / host tissues.
- On arrival to a suitable substrate (crop, seed surface, wounded tissue), conidia attach, adhere and germinate forming germ tubes.
- Germ tubes elongate into hyphae, penetrate surface layers / tissues, and colonization is established with vegetative growth.
- As colonization proceeds, new conidiophores are produced on the colonized substrate, and conidia are again generated (secondary inoculum).
- Under stress or nearing adverse phase, mycelium may aggregate into new sclerotia, which act as survival/resting structures for next unfavorable period.
- Occasionally, sexual or parasexual recombination may occur (in some Aspergillus species); though in many A. flavus strains asexual cycle predominates.
- The cycle is thus completed when sclerotia or spores remain until next season or until favorable conditions return to initiate germination again.
Genomics Studies of A. flavus
- Aspergillus flavus is closely related to the species A. oryzae, which is used to produce fermented delicacies in Asia.
- The complete genomic sequences of A. flavus and A. oryzae have been determined; each consists of 37 megabases (Mb) organised into eight chromosomes and is predicted to encode approximately 12,000 proteins.
- A. oryzae contains a greater number of genes that code for extracellular hydrolases than A. flavus, but is otherwise remarkably similar.
- Only 43 genes are unique to A. flavus and 129 genes are unique to A. oryzae; 709 genes were found to be polymorphic between the two species.
- Numerous non-aflatoxin-producing A. flavus isolates are associated with crops, and their relationship to A. oryzae, which has been considered a distinct species for a long time, is ambiguous.
- The increased number of genes encoding secretory hydrolytic enzymes, proteins involved in amino acid metabolism, and amino acid/sugar uptake transporters in A. oryzae compared to A. flavus supports the notion that the domestication of A. oryzae as a species better adapted for fermentation resulted in gene expansion.
- A. flavus genomic studies have improved our understanding of secondary metabolism and its regulation.
- 55 putative secondary metabolite clusters, including siderophores and genes involved in the biosynthesis of known A. flavus toxins, have been identified in A. flavus.
- Overproduction of a particular gene product that may be involved in modulating vegetative growth and is flanked by genes encoding a chitin synthase activator and a cell wall glucanase correlates with repression of aflatoxin biosynthesis.
- Comparing aflatoxin-conducive and nonconducive temperatures for growth revealed that expression of the regulatory genes aflR and aflS(J) was unaffected by temperature, indicating that the nonconducive temperature for aflatoxin production affects the stability of a key protein required for biosynthesis.
Pathogenesis of Aspergillus flavus
- Initially, conidia of Aspergillus flavus are inhaled (in case of animal/human) or deposited on plant surface / tissue under field or storage conditions.
- After deposition, the spores are adhered to host surfaces (epithelium / plant cuticle), often mediated by surface proteins / adhesion molecules.
- Germination is triggered when environmental conditions (moisture, temperature, nutrients) become favorable, and germ tubes emerge from spores.
- The germ tube elongates, differentiates hyphae, and then penetration into host tissue or across surface occurs by mechanical pressure / enzymatic degradation.
- Extracellular hydrolytic enzymes (proteases, lipases, amylases etc.) are secreted to degrade host tissues, facilitating colonization, nutrient release and invasion.
- The fungal cell wall integrity / structure is maintained to resist host defenses and environmental stresses (so that hyphae survive).
- Oxidative stress defenses (e.g. catalase, superoxide dismutase) are activated to neutralize reactive oxygen species produced by host immune cells, thus aiding survival
- During colonization, secondary metabolites such as aflatoxins are synthesized by fungal metabolic pathways and may contribute to tissue damage or modulating host response.
- The aflatoxin B₁ compound is capable of forming DNA adducts and interfering with host cell functioning, thereby causing mutagenesis, cytotoxicity and possibly facilitating deeper invasion.
- In immunocompromised hosts, hyphal invasion can proceed into deeper tissues, blood vessels and organs, causing invasive aspergillosis and systemic dissemination.
- In plant pathogenesis, colonization often proceeds in wounded tissues, kernels, or stressed parts (e.g. drought-stressed plants), where host defenses are weakened, making invasion easier.
- Biofilm formation may be involved in persistent colonization, which aids in evasion of host immune responses and creates microenvironments favorable for fungal growth.
- Finally, new conidia are produced on colonized tissues, which are released and dispersed to infect new sites or hosts, thus continuing the pathogenic cycle.
Diversity in A. flavus Populations
- Populations of Aspergillus flavus are diverse and constituted of vegetative compatibility groups (VCGs).
- VCGs limit genetic exchange between VCGs and restrict hyphal fusion to genotypes within the same VCG.
- New VCGs may arise as a result of random mutations at compatibility loci, or through genetic recombination.
- A population of A. flavus from Georgia demonstrates a lengthy history of recombination.
- By rearranging genes, it is possible to produce progeny with a novel genomic structure that is vegetatively incompatible with both parental and sibling strains.
- A population undergoing constant recombination will eventually contain individuals with numerous VCGs.
- A. flavus isolates from distinct VCGs can vary in enzyme production, virulence, and aflatoxin-producing capacity.
- A. flavus has been classified into two sclerotial morphotypes, S (small) and L (large), based on the size disparities of their sclerotia.
- Recent phylogenetic research indicates that these morphotypes diverged independently from an aflatoxin B- and G-producing ancestor.
- S-group isolates produce more sclerotia and fewer conidia than L-group isolates and respond differentially to pH, growth, and differentiation in light and dark environments. The ‘typical’ A. flavus strain L produces fewer sclerotia but more conidia when grown under identical conditions.
Laboratory Diagnosis Methods for Aspergillus flavus
- Direct microscopy is performed on clinical or environmental specimens (tissue, sputum, BAL fluid) using stains such as lactophenol cotton blue, calcofluor white, Gomori methenamine silver or PAS, and morphological features are observed (hyphae, septations, branching etc.).
- Culture (on fungal media such as Sabouraud dextrose agar, Czapek-Dox, MEA etc.) is used to isolate the fungus, and characteristic colony morphology / sporulation patterns are examined to tentatively identify Aspergillus flavus.
- Slide culture or tease-mount / Scotch tape mount is used after growth, so that microscopic structures (conidiophores, vesicles, phialides, conidia) can be visualized directly in situ.
- Biochemical / physiological tests (e.g. growth at different temperatures, tolerance to cycloheximide, utilization of carbon sources) may be used to differentiate between species, though these are less favored nowadays.
- Immunoassays are applied: for example, galactomannan antigen detection (in serum or BAL fluid) is used to detect Aspergillus cell-wall antigen, and (1→3)-β-D-glucan assays may be done to detect fungal cell wall components (though not specific to A. flavus).
- Polymerase chain reaction (PCR) methods are employed (e.g. conventional PCR, nested PCR, multiplex PCR, real-time PCR) to detect Aspergillus DNA sequences (like 18S rRNA, ITS, 28S rRNA) from clinical specimens or culture.
- Quantitative (real-time) PCR is used to estimate fungal load, and species-specific or probe-based assays may allow discrimination of A. flavus among Aspergillus species.
- Next-generation sequencing (NGS) or metagenomic sequencing is used in some advanced labs to detect and identify fungal (Aspergillus) DNA directly from specimens, even when culture is negative.
- In situ hybridization (ISH) and microarray methods are used occasionally to detect fungal nucleic acids within tissue sections, aiding localization and differentiation.
- Restriction fragment length polymorphism (RFLP) or sequencing of PCR amplicons is done to distinguish species / strains and detect genetic variants.
- LAMP (loop mediated isothermal amplification) assays have been developed for rapid detection of Aspergillus DNA in field or resource-limited settings.
- Isolation and antifungal susceptibility testing (AFST) are done on the cultured isolate, using broth microdilution or Etest / disk methods to determine minimal inhibitory concentrations for antifungal drugs.
- Volatile organic compound (VOC) detection or metabolite profiling (e.g. gas chromatography / mass spectrometry) is being explored as non-conventional method to detect fungal presence / activity from breath or environmental air samples.
- Serological tests (antibody detection) may be used in certain contexts (especially in immunocompetent hosts), but are less reliable in immunocompromised patients due to poor antibody responses.
- Cryptic or closely related species are differentiated by sequencing of variable genes (e.g. β-tubulin, calmodulin, ITS, gene markers) from the isolated fungal DNA.
Economic Importance of Aspergillus flavus
- Much of the economic damage is caused via aflatoxin contamination of food and feed crops, which reduces marketability, forces crop rejection, and causes loss of revenue.
- In many countries, export consignments are rejected (or downgraded) when aflatoxin levels exceed regulatory limits, and trade losses are incurred by farmers, exporters, and national economies.
- Post-harvest losses are significant: entire lots of grains, nuts or seeds are sometimes destroyed when contamination is detected, and storage costs / disposal costs are imposed.
- Crop yield is also affected: infected seeds may germinate poorly, seedlings may fail, and plant vigor may be lowered, so yield and quality are reduced.
- In livestock & animal production, contaminated feed leads to mycotoxicoses, reduced weight gain, immunosuppression, lower reproductive performance, higher mortality and veterinary costs.
- In dairy animals, aflatoxin B₁ ingested in feed is metabolized to aflatoxin M₁, which appears in milk and dairy products, and thus contaminated milk loses value or is discarded.
- Public health costs are imposed when humans consume contaminated foods: medical treatment costs, loss of productivity, and health burden (e.g. liver cancer) are indirectly economic losses to society.
- Research, monitoring, testing, regulatory compliance, and mitigation / control strategies all incur substantial costs to governments, industry, and farmers (e.g. aflatoxin testing labs, quality control).
- In some regions, consumer confidence is eroded and market demand is reduced for products perceived as unsafe, which further depresses prices / demand.
- A large fraction of economic loss is “hidden loss,” such as subclinical health effects (lower immune responses, chronic disease) and reduced lifetime productivity in humans and animals, which are difficult to quantify precisely.
- Biocontrol / mitigation strategies (e.g., use of non-toxigenic A. flavus strains, improved postharvest handling, sorting, detoxification) require investment but are costed against the losses prevented.
- In many developing countries, where infrastructure or storage facilities are poor, the relative economic burden of A. flavus contamination is much heavier, and food security is threatened.
- Some economic models estimate that mycotoxin contamination (including that from A. flavus) causes losses in the order of hundreds of millions to billions of USD annually on a global scale.
Treatment of Aspergillus flavus infections
- Initially, antifungal therapy is started as soon as invasive aspergillosis is suspected (before full laboratory confirmation) in high-risk patients.
- The preferred first-line agent is voriconazole, which is recommended for primary treatment of invasive pulmonary aspergillosis (and extrapulmonary disease) in many guidelines.
- In cases where voriconazole is contraindicated or cannot be tolerated, lipid formulations of amphotericin B (or other amphotericin derivatives) are used as alternatives.
- Combination therapy (e.g. a mold-active triazole + an echinocandin) may be considered in critically ill or high-risk patients, or when resistance / treatment failure is suspected.
- Duration of antifungal therapy is typically 6–12 weeks or more, depending on site of disease, immune status, and response to therapy.
- Surgical intervention (debridement / resection) is often required in localized disease (e.g. fungal sinusitis, cutaneous lesions, localized abscess) to remove necrotic tissue and reduce fungal burden.
- In many cases, underlying immunosuppression or risk factors are reduced (if possible), e.g. by reducing immunosuppressive drugs, improving neutrophil counts, giving granulocyte-stimulating factors, etc.
- Therapeutic drug monitoring (TDM) is often used (especially for voriconazole) to ensure adequate drug levels and avoid toxicity.
- In refractory or salvage settings, switching antifungal class (e.g. to posaconazole, isavuconazole, or other agents) is considered, or combination regimens are modified.
- For central nervous system aspergillosis, voriconazole is strongly recommended, and lipid amphotericin B is reserved for those intolerant or refractory to voriconazole.
- In ocular / keratitis infection by A. flavus, topical antifungals (for example voriconazole eye drops, natamycin) are used along with systemic therapy and sometimes surgical measures.
- In cases of persistent or chronic disease, long-term suppressive therapy may be needed, and regular imaging / clinical follow-up is done to assess response or relapse.
Prevention and Control
- In high-risk patients, airborne spore exposure is minimized by use of HEPA-filtered rooms, positive pressure ventilation, and strict environmental control in hospitals.
- In hospital renovation / construction settings, dust control, sealed barriers, negative air machines, and restricted access are implemented so that spores ingress is prevented.
- Prophylactic antifungal therapy (e.g. posaconazole) is used in some immunocompromised patients (e.g. transplant, neutropenia) to reduce risk of invasive disease.
- Use of N95 respirators / masks is recommended when patients with impaired immunity must enter areas with dust, mold, soil exposure or construction zones.
- In endemic or agricultural settings, pre-harvest control is emphasized including crop rotation, proper irrigation, minimizing insect / pest damage, and resistant varieties.
- After harvest, drying of grains / seeds to safe low moisture (e.g. < 11–12 %) is enforced so that fungal growth / aflatoxin production is prevented.
- During storage, temperature control (cooling), aeration, low humidity and exclusion of moisture ingress are applied to limit fungal proliferation.
- Cleaning and sanitation of storage facilities (removal of broken / damaged kernels, dust, old residues) are done so that inoculum load is reduced.
- Use of non-aflatoxigenic strains of A. flavus as biocontrol agents is applied: these harmless strains compete with toxigenic ones and reduce aflatoxin contamination.
- Physical / chemical treatments (e.g. irradiation, low oxygen atmospheres, certain antifungal compounds) are used to inactivate A. flavus or suppress toxin production in stored commodities.
- Monitoring / testing (aflatoxin assays, fungal counts, moisture sensors) is regularly performed to detect early contamination / risk and initiate corrective actions.
- In immunocompromised persons, reduction of immunosuppression (if possible) and vigilant surveillance / early diagnosis are practiced to prevent full-blown infection.
- Education and training of staff, farmers, grain handlers, hospital workers are emphasized so that awareness of risk, good practices, early detection and control are fostered.
- In hospital settings, adherence to infection control protocols (ventilation, air filtration, cleaning, monitoring) is strongly enforced, since outbreaks of aspergillosis have been traced to lapses.
FAQ
What is Aspergillus flavus?
Aspergillus flavus is a species of fungus that is commonly found in soil, decaying vegetation, and agricultural crops, particularly in warm and humid environments. It is known to produce a carcinogenic mycotoxin called aflatoxin, which can contaminate food crops and cause serious health problems in humans and animals.
What is the main concern regarding Aspergillus flavus?
The main concern regarding Aspergillus flavus is its ability to produce aflatoxins, which are potent carcinogens that can cause liver cancer and other health problems in humans and animals if consumed in contaminated food products.
What type of diseases can Aspergillus flavus cause in humans?
Aspergillus flavus can cause a range of respiratory diseases, including aspergillosis, which is an infection of the lungs or other organs. It can also cause allergic reactions in some people.
How is Aspergillus flavus diagnosed?
Aspergillus flavus infections can be diagnosed through a combination of clinical examination, radiological imaging, and laboratory tests, such as culture and microscopy of sputum, bronchoalveolar lavage (BAL) fluid, or other body fluids.
What is the treatment for Aspergillus flavus infections?
Treatment for Aspergillus flavus infections typically involves the use of antifungal medications, such as voriconazole or amphotericin B, administered either orally or intravenously. In some cases, surgical intervention may also be required.
How can Aspergillus flavus be prevented?
Preventing Aspergillus flavus contamination requires a combination of measures, including proper storage and handling of food products, proper ventilation in agricultural and industrial settings, and the use of fungicides and other preventive measures in crops and agricultural fields.
Where is Aspergillus flavus commonly found?
Aspergillus flavus is commonly found in soil, decaying vegetation, and agricultural crops, particularly in warm and humid environments. It is often found in crops such as peanuts, maize, and cottonseed.
What are the key features of Aspergillus flavus growth on culture media?
Aspergillus flavus can be readily distinguished from other Aspergillus species by its lack of growth at 5°C and rapid growth at both 25 and 37°C, and by the production of a bright yellow-green conidial coloration when cultured on malt extract agar (MEA) or Czapek yeast extract agar (CYA).
What factors affect the growth of Aspergillus flavus?
Several factors can affect the growth of Aspergillus flavus, including temperature, moisture, pH, radiation, pressure, and electromagnetic fields.
Can Aspergillus flavus be beneficial in any way?
While Aspergillus flavus is primarily known for its ability to produce aflatoxins, it also has some beneficial uses. It can be used in the production of certain enzymes, such as amylases and proteases, and has potential applications in bioremediation and other environmental processes.
- Dobson, A. D. W. (2002). ASPERGILLUS FLAVUS. Encyclopedia of Dairy Sciences, 116–122. doi:10.1016/b0-12-227235-8/00031-6
- Jackson, S. A., & Dobson, A. D. W. (2016). Yeasts and Molds: Aspergillus flavus. Reference Module in Food Science. doi:10.1016/b978-0-08-100596-5.01086-6
- Bhatnagar, D., Cleveland, T. E., & Payne, G. A. (1999). ASPERGILLUS | Aspergillus Flavus. Encyclopedia of Food Microbiology, 72–79. doi:10.1006/rwfm.1999.0065
- Bhatnagar, D., Ehrlich, K. C., Moore, G. G., & Payne, G. A. (2014). ASPERGILLUS | Aspergillus flavus. Encyclopedia of Food Microbiology, 83–91. doi:10.1016/b978-0-12-384730-0.00012-4
- Hedayati MT, Pasqualotto AC, Warn PA, Bowyer P, Denning DW. Aspergillus flavus: human pathogen, allergen and mycotoxin producer. Microbiology (Reading). 2007 Jun;153(Pt 6):1677-1692. doi: 10.1099/mic.0.2007/007641-0. PMID: 17526826.
- https://www.cifr.ncsu.edu/aflavus/
- https://en.wikipedia.org/wiki/Aspergillus_flavus
It is interesting to note the specific requirements for the growth of A. flavus. Considering these factors, there could be potential strategies for controlling its growth in certain environments. For instance, regulating temperature, water activity, and pH could be used to limit the growth of this fungus in food storage or processing facilities. Additionally, exploring the effect of light exposure, particularly UV light, could offer insights into new methods for preventing aflatoxin production. Further research in these areas could lead to the development of more effective approaches for managing A. flavus contamination.
This is a comprehensive overview of Aspergillus flavus and its pathogenicity in both plants and animals. It’s alarming how this fungus can remain dormant and unnoticed until postharvest storage or transport. The fact that it produces mycotoxins that can be harmful to mammals is also concerning. I appreciate the information about its opportunistic pathogenicity in humans and animals with weakened immune systems. This article highlights the importance of understanding and preventing the spread of A. flavus for public health and food safety.
What are some of the ways that Aspergillus flavus populations can differ and how does this impact their enzyme production, virulence, and aflatoxin-producing ability?
Aspergillus flavus is a species of fungi that can be found in soil, decaying vegetation, and crops such as corn, peanuts, and cottonseed. While some strains of A. flavus are benign, others are capable of producing aflatoxins, which are potent carcinogenic compounds that can contaminate food and feed.
Genetic diversity: A. flavus is a highly diverse species, with variations in its genome leading to different strains and subpopulations. These genetic differences can impact the fungi’s virulence and ability to produce aflatoxins.
Geographical location: A. flavus populations can vary based on the geographic location where they are found. Different environments can result in different microbial communities and nutrients available for the fungi to thrive on, which can impact their enzyme production and aflatoxin-producing ability.
Host species: A. flavus can infect various plant species, and the different plant species can influence the growth and metabolism of the fungi. This can result in differences in their enzyme production and ability to produce aflatoxins.
Environmental conditions: Temperature, humidity, and other environmental factors can impact A. flavus growth and aflatoxin production. For example, higher temperatures and increased moisture levels can promote aflatoxin production.
The differences in A. flavus populations can have significant impacts on their enzyme production, virulence, and aflatoxin-producing ability. For example, some strains of A. flavus are highly virulent and can cause disease in plants and animals, while others are less harmful. The ability to produce aflatoxins is also highly variable, with some strains capable of producing high levels of toxins while others produce little or none.
Additionally, the enzyme production of A. flavus can vary depending on the strain, host, and environmental conditions. A. flavus produces several enzymes that are involved in the degradation of plant material, such as cellulases, hemicellulases, and pectinases. These enzymes are important for the fungi to obtain nutrients from their host and play a critical role in the infection process. The expression of these enzymes can impact the severity of the disease and the ability of the fungi to infect new hosts.
In conclusion, the differences in A. flavus populations can have significant impacts on their enzyme production, virulence, and aflatoxin-producing ability. Understanding these differences can help researchers develop strategies for controlling the spread of the fungi and preventing aflatoxin contamination in food and feed.