What is Agrobacterium?
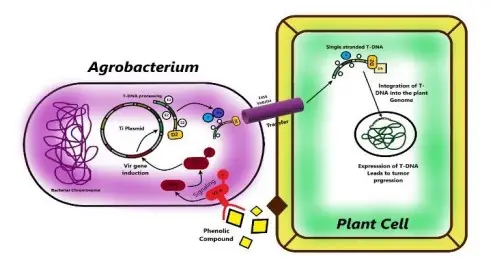
There are many types of Gram-negative bacteria in the genus Agrobacterium. They can be harmful to plants and are also useful for genetic engineering. It causes tumours in plants by moving a piece of DNA known as T-DNA from its Ti or Ri plasmid into the plant genome. This causes cells to divide too quickly and form tumours. This group has species like Agrobacterium tumefaciens, which is the most studied because it causes crown gall disease, and Agrobacterium rhizogenes, which is known for hairy root disease.
Agrobacterium’s ability to move T-DNA is used as a transformation tool to add genes that scientists want to plants. This makes it very important in biotechnology. The host range of Agrobacterium is affected by both bacterial factors, like virulence genes and plasmid types, and plant factors, like how easily they get infected and whether they have receptors.
The Ti (tumor-inducing) and Ri (root-inducing) plasmids that Agrobacterium carries are what make it so different. These plasmids change the symptoms of the disease and the types of hosts that it can infect. Agrobacterium species are found all over the world and can infect many different types of dicotyledonous plants, including fruit trees and ornamental plants.
The main process is the transfer of T-DNA from a plasmid into the plant, which changes its genes and causes tumours to form. Agrobacterium are rod-shaped, move with flagella, and can live in both aerobic and anaerobic environments. They do best in soil and plant-associated environments.
Factors affecting Agrobacterium-mediated Gene Transfer
- Plant Genotype -The genetic composition of a plant plays a crucial role in determining the efficiency of transformation processes. Specific genotypes demonstrate a greater susceptibility to Agrobacterium infection, whereas others may exhibit increased resistance to such interactions.
- Explant Type and Condition -The selection of explant type, such as immature embryos, callus, or leaf discs, along with its physiological condition, plays a pivotal role in the success of transformation processes. Explants derived from tissues that are actively dividing generally demonstrate superior transformation rates.
- Co-cultivation Medium – The co-cultivation medium’s composition, which encompasses the presence of essential nutrients and plant growth regulators, plays a critical role in influencing the survival and regeneration of transformed cells. Additionally, the inclusion of certain additives, such as coconut water, has been demonstrated to improve transformation efficiency.
- Inoculation and Co-cultivation Duration – The timing of Agrobacterium inoculation and the duration of subsequent co-cultivation with plant tissues are critical factors. Optimal durations facilitate adequate T-DNA transfer while simultaneously reducing the risk of bacterial overgrowth, which may result in necrosis.
- Acetosyringone Concentration – Acetosyringone, classified as a phenolic compound, serves a critical role as an inducer of virulence genes in Agrobacterium. The inclusion of this compound in the co-cultivation medium has been shown to markedly improve transformation efficiency.
- Agrobacterium Strain Selection -The selection of Agrobacterium strain is a critical factor influencing the success of transformation processes. Strains characterised by hypervirulence, such as EHA101, are frequently employed owing to their superior capacity for T-DNA transfer.
- Antibiotic Selection Pressure – The application of selective antibiotics in the regeneration medium is crucial for the survival of transformed cells. Nonetheless, it is important to note that high concentrations of antibiotics may impede plant growth and regeneration.
- Regeneration Protocols -Regeneration protocols play a critical role in the development of whole plants from transformed cells. It is imperative that these protocols are optimised for each specific plant species to guarantee successful plant development.
- Temperature and Environmental Conditions – The efficiency of transformation is significantly influenced by environmental factors, including temperature and light conditions, particularly during the co-cultivation and regeneration stages. Therefore, it is essential to maintain optimal conditions to achieve successful transformation.
- Bacterial Density and Preparation -The concentration of Agrobacterium cells utilised for inoculation requires meticulous regulation, as both excessive and insufficient bacterial densities can adversely influence the outcomes of transformation.
- Wounding of Plant Tissues -The mechanical wounding of plant tissues serves as a means to facilitate Agrobacterium infection. Nevertheless, it is important to note that excessive wounding may result in tissue necrosis, which in turn can diminish the efficiency of transformation.
- Use of Surfactants -The application of surfactants, such as Silwet L-77, has been shown to facilitate bacterial attachment to plant tissues, thereby enhancing transformation rates. It is essential to optimise their concentration to prevent any potential phytotoxic effects.
- Explants Pre-culture Duration -The duration of pre-culturing explants prior to inoculation plays a significant role in enhancing their responsiveness to Agrobacterium. It is important to note, however, that extended pre-culture periods may result in a diminished capacity for regeneration.
- Bacterial Growth Phase – The growth phase of Agrobacterium at the time of inoculation plays a significant role in influencing transformation efficiency. It has been observed that bacteria in the late log phase generally exhibit a higher competency for T-DNA transfer.
- Use of Helper Plasmids – The incorporation of helper plasmids serves to augment virulence functions, thereby facilitating the transfer of T-DNA. Nonetheless, it is imperative to manage their presence judiciously to mitigate any potential adverse effects.
- Selection Marker Systems – The selection of appropriate markers, such as antibiotic resistance genes, is critical and must align with the specific plant species and regeneration system employed. This compatibility is essential for the successful survival of transformed cells.
- Callus Induction and Maintenance -The process of callus induction and maintenance is essential for achieving successful transformation. It is imperative to optimise both the composition of the induction medium and the intervals for subculturing.
- Opine Production – The generation of opines by transformed plant cells serves as a nutrient source for Agrobacterium. Furthermore, the existence of opines may affect the selection pressure encountered during the transformation process.
- Contamination Control -Contamination control is of paramount importance in laboratory settings. The implementation of strict aseptic techniques is crucial to mitigate the risk of contamination by extraneous microorganisms. Such contaminants can engage in competitive interactions with Agrobacterium, thereby adversely influencing the success of transformation processes.
- Regeneration from Transformed Cells -The process of regeneration from transformed cells represents a fundamental aspect of plant biotechnology. Achieving successful regeneration into complete plants necessitates the optimisation of protocols to guarantee elevated regeneration rates from the transformed tissues.
Why transform plants using Agrobacterium?
Agrobacterium-mediated gene transfer is a preferred method for transforming plants for several reasons:
- High transformation efficiency: Agrobacterium-mediated gene transfer can result in high transformation efficiencies, meaning that a large number of plants can be efficiently transformed with the desired gene(s).
- Precise and targeted gene insertion: Agrobacterium-mediated gene transfer allows for the precise and targeted insertion of new genes into specific sites in the plant genome, resulting in a more stable and predictable gene expression.
- Versatility: Agrobacterium-mediated gene transfer can be used to introduce a wide range of genes into plant genomes, including genes from other species, synthetic genes, and regulatory elements.
- Safe and natural process: Agrobacterium-mediated gene transfer is a natural process that occurs in nature, and does not require the use of harsh chemicals or physical methods that can damage plant cells.
- Compatibility with tissue culture techniques: Agrobacterium-mediated gene transfer is compatible with tissue culture techniques, which allows for the regeneration of whole plants from transformed cells.
Overall, Agrobacterium-mediated gene transfer is a reliable and effective method for introducing new genes into plant genomes, which has led to the development of genetically modified crops with improved traits and increased yields.
Principle of Agrobacterium-mediated Gene Transfer
- Agrobacterium transfers its T-DNA efficiently into host plant cells.
- The process consists of two primary components: T-DNA characterised by 25 base pair repeats concluding at the T-region, and the virulence (vir) region, which encompasses seven significant loci.
- During the process of infection, bacteria facilitate the transfer of a segment of plasmid DNA into plant cells.
- The Ti (tumor-inducing) plasmid is characterised by its ability to integrate into the nuclear genome of the plant.
- The integrated DNA facilitates the expression of genes that modify the hormonal equilibrium within the plant system.
- The bacteria generate enzymes that facilitate the synthesis of opines, which function as nutritional sources for the bacteria.
- The fundamental components of bacteria encompass T-DNA located on the Ti plasmid, as well as regions associated with virulence (vir), conjugation (con), and the origin of replication (ori).
- Infection initiates when bacteria infiltrate through damaged areas of the plant.
- Injured plant cells initiate the release of phenolic acetosyringone (AS), which subsequently enhances the binding affinity of bacteria.
- AS initiates the activation of the bacterial VirA protein, which subsequently phosphorylates and activates VirG.
- The activation of VirG leads to the expression of additional virulence genes.
- VirD plays a crucial role in the stimulation of T-strand production, resulting in the formation of a single-stranded copy of T-DNA.
- VirD2 exhibits a covalent binding affinity for the 5’ terminus of the T-strand, which serves as the leading edge during the transfer process.
- The VirE2 and VirB proteins interact with the T-strand, resulting in the formation of the T-complex.
- The transport of the T-complex into the plant nucleus is facilitated by nuclear targeting signals derived from Vir proteins.
- T-DNA integrates into the plant genome in a random manner, frequently occurring in transcriptionally active or repetitive regions through the process of recombination.
- The factors encoded by plants that are involved in the transfer of T-DNA are still largely uncharacterized.
Requirements for Agrobacterium-mediated Gene Transfer
- Sterile 50 ml plastic tubes – Used for bacterial culture and transformation procedures.
- Autoclave – Sterilizes glassware, media, and equipment to prevent contamination.
- Controlled Tissue Culture Rooms at 25°C with 16/8 hr light/dark period – Maintain optimal conditions for plant tissue growth and regeneration.
- Shaker Incubator – Provides controlled shaking for bacterial cultures at specific temperatures.
- Vacuum pump – Assists in removing air during vacuum infiltration of plant tissues.
- Laminar hood for tissue culture – Provides a sterile environment for handling plant tissues.
- Glassware (Beakers, cylinders, Petri dishes, Duran bottles, and Flasks) – Essential for preparing and storing media and solutions.
- Filter paper – Used for sterilizing solutions by filtration.
- Parafilm – Seals containers to prevent contamination.
- Forceps and Scalpel – Tools for excising plant explants under sterile conditions.
- Pipettes – For accurate measurement and transfer of liquids.
- Centrifuge – Separates components in a mixture based on density.
- Spectrophotometer – Measures the absorbance of bacterial cultures to estimate cell density.
- Tissue culture vessels – Containers for growing plant tissues.
- Surgical blades – Used for precise cutting of plant tissues.
- Explant (Stems, embryo, cotyledons, or other tissues) – Plant material used for transformation.
- Agrobacterium strain – Bacterial species used for gene transfer; commonly used strains include EHA101, EHA105, and LBA4404.
- 13% Sodium hypochlorite – Used for surface sterilization of plant materials.
- B5 Medium – Basal medium for plant tissue culture, providing essential nutrients.
- Agar – Solidifying agent for media preparation.
- Tryptone – Source of nitrogen and growth factors for bacterial culture.
- Yeast Extract – Provides vitamins and growth factors for bacterial culture.
- Sodium Chloride – Maintains osmotic balance in media.
- 35% Hydrochloric acid – Adjusts pH of solutions.
- Sterile distilled water – Used for preparing solutions and media.
- 75% Ethanol – Used for sterilization of surfaces and tools.
- Sucrose – Provides carbon source for plant tissue growth.
- Abscisic Acid – Regulates plant growth and stress responses.
- Rifampicin – Antibiotic used to select for transformed bacteria.
- Kanamycin monosulfate – Antibiotic used for selecting transformed plant tissues.
- Gellan gum powder – Alternative gelling agent for media preparation.
- PCR primer star Mix – Contains primers for amplifying target DNA sequences.
- Carbenicillin disodium salt – Antibiotic used to eliminate Agrobacterium after transformation.
Required Media and their Preparation for Agrobacterium-mediated Gene Transfer
Co-cultivation Medium (CCM) – Contains ½ Gamborg’s B5 medium, 2% sucrose, 0.5 g/L MES, 1.67 mg/L BAP, 0.25 mg/L GA3, 40 mg/L acetosyringone, and 0.7% agar; pH adjusted to 5.4.
Infection Medium (IM) – Comprises ½ Gamborg’s B5 medium, 2% sucrose, 0.5 g/L MES, 1.67 mg/L BAP, 0.25 mg/L GA3, 40 mg/L acetosyringone; pH adjusted to 5.4.
Shoot Induction Medium (SI) – Formulated with Gamborg’s B5 medium, 2% sucrose, 0.5 g/L MES, 1.67 mg/L BAP, 0.25 mg/L GA3, 40 mg/L acetosyringone, 0.7% agar; pH adjusted to 5.4.
Shoot Elongation Medium (SE) – Contains MS medium, 2% sucrose, 0.5 g/L MES, 1.67 mg/L BAP, 0.25 mg/L GA3, 40 mg/L acetosyringone, 0.7% agar; pH adjusted to 5.4.
Rooting Medium (RM) – Comprises MS medium, 2% sucrose, 0.5 g/L MES, 1.67 mg/L BAP, 0.25 mg/L GA3, 40 mg/L acetosyringone, 0.7% agar; pH adjusted to 5.4.
Agrobacterium Culture Medium – Prepared with 5 g yeast extract, 10 g tryptone, 10 g NaCl, 15 g agar; pH adjusted to 7.0. Microbe Notes
Co-cultivation Medium (Alternative) – Formulated with ½ Gamborg’s B5 medium, 2% sucrose, 0.5 g/L MES, 1.67 mg/L BAP, 0.25 mg/L GA3, 40 mg/L acetosyringone, 0.7% agar; pH adjusted to 5.4.
Shoot Induction Medium (Alternative) – Comprises Gamborg’s B5 medium, 2% sucrose, 0.5 g/L MES, 1.67 mg/L BAP, 0.25 mg/L GA3, 40 mg/L acetosyringone, 0.7% agar; pH adjusted to 5.4.
Rooting Medium (Alternative) – Contains MS medium, 2% sucrose, 0.5 g/L MES, 1.67 mg/L BAP, 0.25 mg/L GA3, 40 mg/L acetosyringone, 0.7% agar; pH adjusted to 5.4.
Selection Medium – Formulated with Gamborg’s B5 medium, 2% sucrose, 0.5 g/L MES, 1.67 mg/L BAP, 0.25 mg/L GA3, 40 mg/L acetosyringone, 0.7% agar; pH adjusted to 5.4.
Antibiotic Selection Medium – Prepared with Gamborg’s B5 medium, 2% sucrose, 0.5 g/L MES, 1.67 mg/L BAP, 0.25 mg/L GA3, 40 mg/L acetosyringone, 0.7% agar; pH adjusted to 5.4.
Regeneration Medium – Comprises Gamborg’s B5 medium, 2% sucrose, 0.5 g/L MES, 1.67 mg/L BAP, 0.25 mg/L GA3, 40 mg/L acetosyringone, 0.7% agar; pH adjusted to 5.4.
Rooting Medium (Final) – Formulated with MS medium, 2% sucrose, 0.5 g/L MES, 1.67 mg/L BAP, 0.25 mg/L GA3, 40 mg/L acetosyringone, 0.7% agar; pH adjusted to 5.4.
Co-cultivation Medium (Soybean) – Prepared with ½ Gamborg’s B5 medium, 2% sucrose, 0.5 g/L MES, 1.67 mg/L BAP, 0.25 mg/L GA3, 40 mg/L acetosyringone, 0.7% agar; pH adjusted to 5.4.
Shoot Induction Medium (Soybean) – Comprises Gamborg’s B5 medium, 2% sucrose, 0.5 g/L MES, 1.67 mg/L BAP, 0.25 mg/L GA3, 40 mg/L acetosyringone, 0.7% agar; pH adjusted to 5.4.
Rooting Medium (Soybean) – Contains MS medium, 2% sucrose, 0.5 g/L MES, 1.67 mg/L BAP, 0.25 mg/L GA3, 40 mg/L acetosyringone, 0.7% agar; pH adjusted to 5.4.
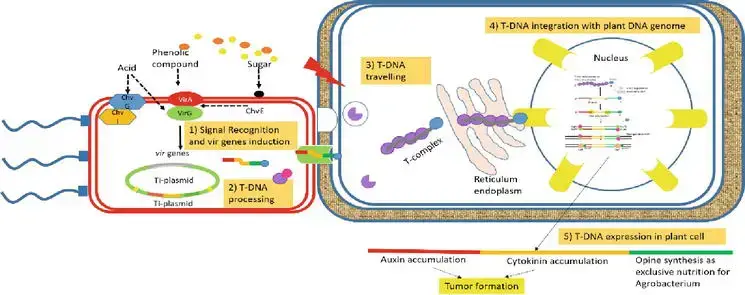
Agrobacterium-mediated transformation mechanism (Natural pathogenesis of A. tumefaciens)
AMT is a complicated mechanism, which includes
- signal recognition from plant host to A. tumefaciens,
- T-DNA processing,
- T-DNA traveling in plant host cell,
- T-DNA integrating to plant host genome, and
- expression of T-DNA in the plant host cell.
The mechanism of T-DNA transfer is facilitated by a set of virulent genes located on Ti-plasmid borne [approximately 35 virulent genes grouped in at least 8 operons, virA, virB, virC, virD, virE, virF, virG, and virH, encoding VirA, VirB, VirC, VirD, VirE, VirF, VirG, and VirH protein, respectively ], apart from T-DNA, whereas others are on chromosome (chromosomal virulent genes—chv).
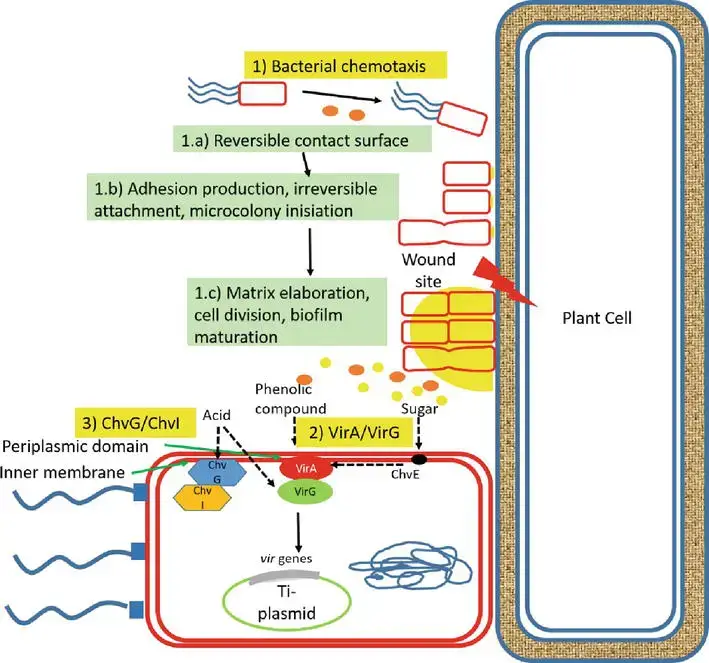
1. Signal recognition
- Large plant-derived chemicals, including organic acid compounds (pH 5.0–5.8) as routinely released chemicals and phenolic compounds as wound-releasing chemicals, interact with Agrobacterium when exposed to the bacteria.
- Three systems are involved in the recognition of A. tumefaciens by plant cells. First, phenolic chemicals, such as acetosyringone and -hydroxy acetosyringone, released from fresh wound sites of plants promote bacterial chemotaxis.
- Initially, bacteria interact with the plant cell surface in a reversible manner, encouraging adhesion synthesis that results in adhesion via unipolar polysaccharide (UPP)-dependent polar attachment and UPP-independent attachment.
- This irreversible surface attachment establishes a site for the creation of multicellular biofilms, matrix elaboration, cell division, biofilm maturation, and the dispersal of “buddy daughter” cells.
- Second, host signal molecules are also detected by transmembrane protein receptors (VirA) in the periplasmic region of bacterial cells, which activate the phosphorylation of the positive regulatory protein VirG. Thirdly, the chromosomal virulent gene-encoded protein ChvG/ChvI detects acidity from sugar, which also activates the basal production of virG.
- The phosphorylation of VirG activates an additional virulence gene. Monosaccharides augment the signaling mechanism by attaching to ChvE and then collaborating with VirA.
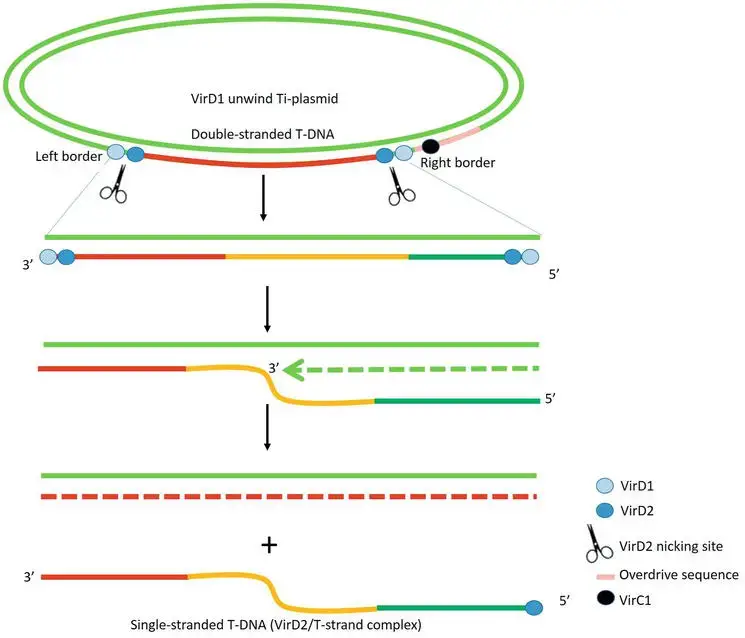
2. T-DNA processing
- The process of removing T-DNA from Ti-plasmids is dependent on the endonucleases VirD1 and VirD2. The 25 base pair border sequences on the bottom strand of T-DNA serve as nicking sites for VirD1 and VirD2. Site-specific VirD1 helicase unwinds double-stranded T-DNA.
- The nuclease VirD2 breaks the bottom strand of T-DNA from the right and left border, resulting in T-strand single-stranded linear DNA.
- VirD2 then covalently caps the 5′ end of T-strand at the right border, producing the complex VirD2/T-strand. The 3′ end of the nicked right border serves as a priming point for the regeneration of the bottom strand of T-DNA.
- VirC1 increases the number of T-strand molecules by binding the “overdrive” sequence near the right border of T-DNA via its C-terminal ribbon-helix-helix DNA binding fold.
- The appropriate proportion of the 25 kb terminal sequence of T-DNA determines the DNA transfer director.
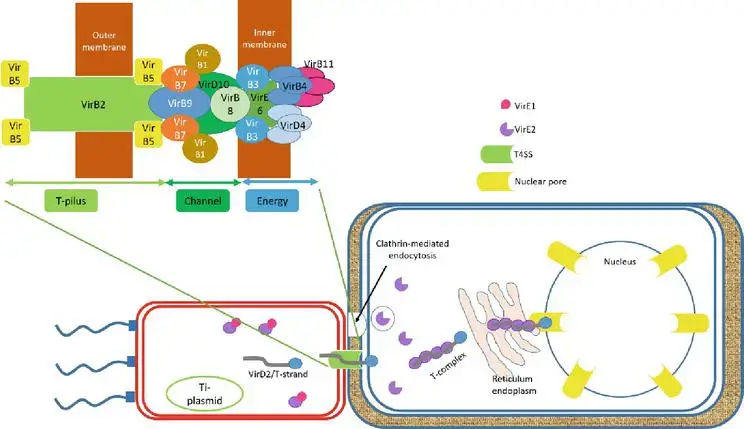
3. T-DNA traveling
- The role of VirD2 and VirE2 cannot be separated from the T-DNA migrating to the host. Both VirD2 and VirE2 possess the C-terminal nuclear localization signal (NLS) sequence that guided VirD2/T-strand to the nucleus of the host cell.
- VirD2/T-strand, a rod-like structure, exits bacterial cells via the Ti-pilus, type IV secretion system (T4SS), which is composed of 11 VirB and VirD4 proteins. By clathrin-mediated endocytosis, the hydrophilic protein VirE2 accumulates in the bacterial cytoplasm and is translocated into the host cell.
- In addition to aiding in the transport of the T-strand, VirE2 in the host cytoplasm forms the noncovalent VirD2/T-strand/VirE2 (Ti-complex) complex to shield the T-strand from nuclease digestion. Ti-complex is transported to the nucleus of the plant via the endoplasmic reticulum network in the plant cytoplasm.
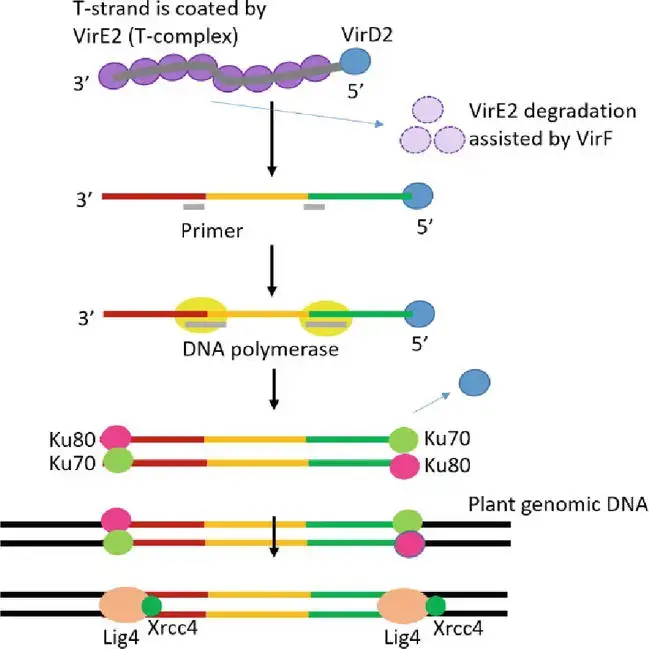
4. T-DNA integration
- T-DNA integration followed by transgene expression is the final and most essential step in Agrobacterium-mediated genetic transformation. In actuality, the molecular mechanism of T-DNA integration into the genome of the host plant is not yet entirely understood.
- T-DNA integration occurs at random places, not preferentially in sections of the plant genome that are transcriptionally active or hypomethylated. Certain essential genes for the T-DNA integration process are restricted to those involved in chromatin formation and histone modification.
- Through binding to the bZIP transcription factor VirE2-interacting protein 1, VirE2 may be involved in T-strand localization to chromatin (VIP1).
- VIP1 mediates the connection between VirE2/single-strand DNA and mononucleosomes. As the T-complex arrives in the plant nucleus, its protein component should be destroyed by the ubiquitin-proteasome system in order to reveal the T-strand. VirF facilitates disassembly of the T-complex and degradation of VirE2.
- As VirD2 lacks ligation activity, it is unlikely that T-strands will link directly with the host genome. It is possible that the host DNA polymerase duplicates the T-strand to form a double-stranded T-DNA, which then fuses with site breaks in the DNA of the host plant caused by environmental stress or regular metabolic activities.
- Non-homologous end joining (NHEJ) as a method of repairing broken double-stranded DNA has been hypothesized as the primary process of bacterial plant DNA integration. NHEJ requires minimal or no sequence homology on the damaged region, despite the fact that there is microhomology between the T-DNA and the integration locations on the host chromosome.
5. T-DNA expression
- There are two probable outcomes for bacterial T-DNA that integrates with plant cells. Initially, the T-DNA is expressed at multiple levels. Second, the T-DNA cannot be expressed because it is merely integrated. Depending on the species, transgenic expression might range from very high to completely silent.
- The buildup of auxin and cytokinin is caused by the expression of auxin and cytokinin coding genes in T-DNA. Abnormal ratios of phytohormones cause plant cells to proliferate uncontrollably, resulting to tumor development.
- The expression of opine synthesis coding genes results in the production of opine, the kind of which varies on the bacterial strain and is an exclusive source of nourishment for Agrobacterium.
- In immature tissue, swelling is detected on the fourth or fifth day after bacterial inoculation, is well-developed by one month, and grows rapidly for several months until it reaches two inches in diameter.
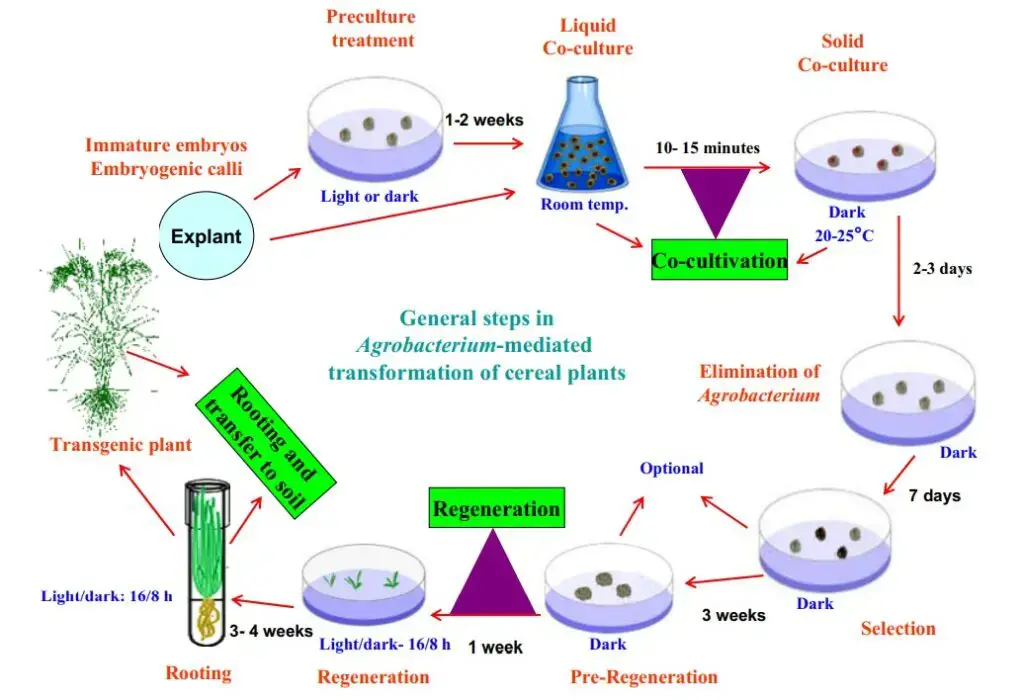
Agrobacterium-mediated transformation protocol
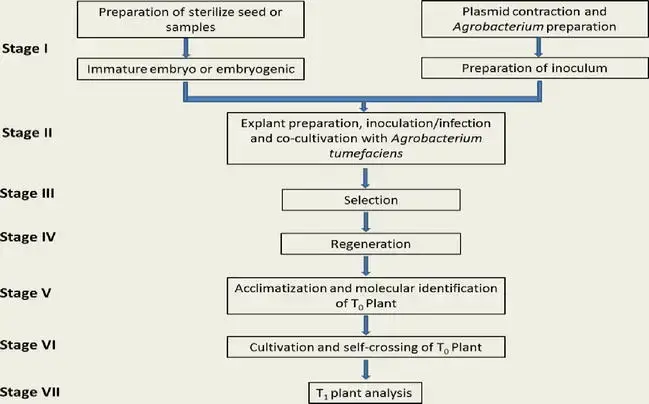
Here is the simplified steps of Agrobacterium-mediated transformation protocol
- Seed Sterilization and Germination
- Seeds are sterilized with chlorine gas for 1–2 hours, then soaked in water for 2 hours at room temperature.
- Seed coats are removed using forceps, sterilized with 75% ethanol for 30 seconds, and rinsed with 3% sodium hypochlorite.
- Sterilized seeds are placed on germination medium and incubated at 28°C in the dark for 2 days.
- Inoculum Preparation
- A single Agrobacterium colony is inoculated into 2 ml of LB medium containing rifampicin and kanamycin.
- The culture is incubated at 28°C with shaking.
- After incubation, the culture is centrifuged at 4000g for 10 minutes, the supernatant is discarded, and the pellet is resuspended in 1 ml of MS liquid medium.
- Explant Preparation
- Germinated seeds are removed from the germination medium and placed on sterile Petri plates with filter paper.
- The radicle is excised, and the seed is halved to remove cotyledons and endosperm.
- Cotyledons are separated and placed in MS liquid medium.
- Agrobacterium Infection
- Cotyledons are transferred to the Agrobacterium suspension and gently shaken.
- The beaker is covered and placed in a desiccator connected to a vacuum pump.
- Two vacuum infiltration sessions are performed, each lasting 5 minutes.
- Co-cultivation
- Sterile filter paper is placed on co-cultivation medium.
- Infected cotyledons are placed on the filter paper with the adaxial surface facing up.
- Petri plates are sealed and incubated in the dark at 28°C for 2 days.
- Shoot Initiation
- Cotyledons are transferred to shoot induction medium containing kanamycin and carbenicillin.
- Plates are incubated under light at 25°C for 2–3 weeks.
- Regeneration
- Shoots are excised and placed on rooting medium.
- Each vessel contains 3–4 shoots and is incubated under light at 25°C for 1–2 weeks.
- If no roots appear, shoots are transferred to fresh rooting medium.
- Acclimatization
- After root development, flask covers are loosened and plants are incubated at 25°C for 3 days.
- Plants are removed from the medium, washed with running water, and transferred to pots with wet compost.
- Pots are covered with zip bags and incubated under light at 25°C for 1–2 weeks.
- Once acclimatized, zip bags are removed, and plants are watered.
Step 1 – Sterilization and Germination
Seed Sterilization
- Seeds are sterilized using chlorine gas for 1–2 hours, followed by soaking in water for 2 hours at room temperature.
- Seed coats are removed using forceps, sterilized with 75% ethanol for 30 seconds, and rinsed with 3% sodium hypochlorite.
- Sterilized seeds are placed on germination medium and incubated at 28°C in the dark for 2 days.
Seed Germination
- Germinated seeds are removed from the germination medium and placed on sterile Petri plates with filter paper.
- The radicle is excised, and the seed is halved to remove cotyledons and endosperm.
- Cotyledons are separated and placed in MS liquid medium.
- Cotyledons are transferred to the Agrobacterium suspension and gently shaken.
- The beaker is covered and placed in a desiccator connected to a vacuum pump.
- Two vacuum infiltration sessions are performed, each lasting 5 minutes.
- Sterile filter paper is placed on co-cultivation medium.
- Infected cotyledons are placed on the filter paper with the adaxial surface facing up.
- Petri plates are sealed and incubated in the dark at 28°C for 2 days.
Step 2 – Inoculum Preparation
- Put a single Agrobacterium colony in 2 ml of LB medium that has rifampicin and kanamycin in it.
- To help the bacteria grow, shake the culture at 28°C for a few hours.
- When the culture has grown enough, spin it in a centrifuge at 4000g for 10 minutes to make the bacteria settle.
- To wash the cells, throw away the supernatant and put the bacterial pellet back in 1 ml of MS liquid medium.
- Do the washing and centrifugation steps again to make sure all the leftover antibiotics are gone.
- Set the final bacterial suspension to the right optical density (OD600) for infection, which is usually between 0.5 and 1.0.
- The Agrobacterium suspension is now ready to be used to change plants.
Step 3 – Explant Preparation
- Position sterilized seeds in a sterile Petri dish filled with sterile deionized water, ensuring complete submersion of the seeds.
- Envelop the Petri dish with aluminum foil to shield the seeds from light and incubate at ambient temperature for 20 hours.
- In a sterile environment within a laminar flow hood, transfer seeds to a sterile Petri dish.
- Employ a sterile scalpel to create a longitudinal incision along the hilum of each seed to detach the cotyledons.
- Detach the seed coat and sever the embryonic axis from the cotyledons.
- Eliminate any residual axial buds connected to the cotyledonary node.
- Place the prepared half-seed explants into a sterile Petri dish with infection medium.
- Immerse explants in the infection medium and incubate at ambient temperature for 30 minutes, gently agitating periodically.
- Following incubation, eliminate surplus infection medium by delicately tapping the explants against the edge of the Petri dish.
- Transfer explants to the co-cultivation medium with sterile filter paper, positioning the adaxial side of the cotyledons downward.
- Seal the Petri dish with parafilm and incubate at 24°C under an 18-hour light and 6-hour dark photoperiod for 5 days.
Step 4 – Agrobacterium Infection
- Prepare the Agrobacterium suspension by resuspending the bacterial pellet in MS liquid medium to the desired optical density (OD600), typically around 0.5 to 1.0.
- Add 100 µM acetosyringone to the Agrobacterium suspension to enhance virulence gene expression.
- Submerge the prepared explants in the Agrobacterium suspension, ensuring thorough coverage.
- Incubate the explants with Agrobacterium at room temperature for 30 minutes, gently agitating occasionally.
- After incubation, remove excess Agrobacterium suspension by gently blotting the explants on sterile filter paper.
- Proceed to the co-cultivation step promptly to facilitate T-DNA transfer into the plant cells.
Step 5 – Co-cultivation
- Transfer the infected explants to a co-cultivation medium, such as MS or B5 agar, and incubate at 22–25°C for 2–3 days.
- Ensure the co-cultivation medium contains acetosyringone to enhance virulence gene expression in Agrobacterium.
- Maintain the explants in the dark during the co-cultivation period to promote transformation efficiency.
- After co-cultivation, transfer the explants to a selection medium containing appropriate antibiotics or herbicides to eliminate non-transformed cells.
- Monitor the explants for the development of resistant shoots over the following weeks.
- Subculture the resistant shoots onto fresh selection medium as needed to promote further growth and regeneration.
- Once sufficient shoot growth is observed, transfer the shoots to a rooting medium to initiate root development.
- After rooting, acclimate the plantlets to greenhouse conditions for further development.
Step 6 – Shoot Initiation
- After co-cultivation, transfer the explants to a resting medium for 2–4 days to allow recovery and initiation of callus formation.
- Subculture the explants onto a shoot induction medium (SIM) containing appropriate plant growth regulators, such as BAP, and a selection agent like kanamycin to select for transformed cells.
- Incubate the explants in the dark for 2–3 weeks to promote shoot initiation.
- Monitor the explants for the development of shoot buds.
- Once shoot buds are visible, transfer the explants to a shoot elongation medium containing a lower concentration of BAP to promote shoot elongation.
- Maintain the explants under a light/dark photoperiod (e.g., 16 hours light/8 hours dark) at 22–25°C to support shoot growth.
- Continue to subculture the explants onto fresh shoot elongation medium as needed to promote further shoot development.
- Once shoots reach an adequate size, transfer them to a rooting medium containing appropriate auxins to induce root formation.
- After roots have developed, acclimate the plantlets to greenhouse conditions for further growth.
Step 7 – Regeneration
- After shoot initiation, transfer the explants to a shoot elongation medium containing reduced concentrations of cytokinins, such as 6-benzylaminopurine (BAP), to promote shoot growth.
- Maintain the cultures under a 16-hour light/8-hour dark photoperiod at 23–25°C to support shoot development.
- Subculture the explants onto fresh shoot elongation medium every 2–3 weeks to ensure continuous shoot growth.
- Once shoots reach an adequate size, transfer them to a rooting medium containing auxins like indole-3-butyric acid (IBA) to induce root formation.
- After root development, acclimate the plantlets to greenhouse conditions by gradually reducing humidity and transferring them to pots with soil.
- Monitor the acclimatized plants for growth and development to ensure successful regeneration.
Step 8 – Acclimatization
- Remove plantlets from tissue culture vessels and gently wash away residual agar using sterile techniques.
- Trim any damaged or non-viable tissues to promote healthy growth.
- Plant the cleaned plantlets into a suitable substrate, such as a mix of perlite and fine peat, ensuring the roots are adequately supported.
- Place the potted plantlets under a humidity dome or in a controlled environment to maintain high humidity levels (80–90%) for the first 1–2 weeks.
- Provide indirect light or low-intensity grow lights for 12–16 hours daily to support acclimatization without causing stress.
- Maintain temperatures around 22–25°C to facilitate acclimatization.
- Gradually reduce humidity by venting the dome or removing it partially over 7–10 days to help the plantlets adapt to ambient conditions.
- Monitor plantlets for signs of stress, such as wilting or discoloration, and adjust environmental conditions accordingly.
- Once plantlets show new growth and appear acclimated, transplant them into larger pots with standard potting mix for further development.
- Continue to provide appropriate care, including regular watering and suitable light, to promote healthy growth post-acclimatization.
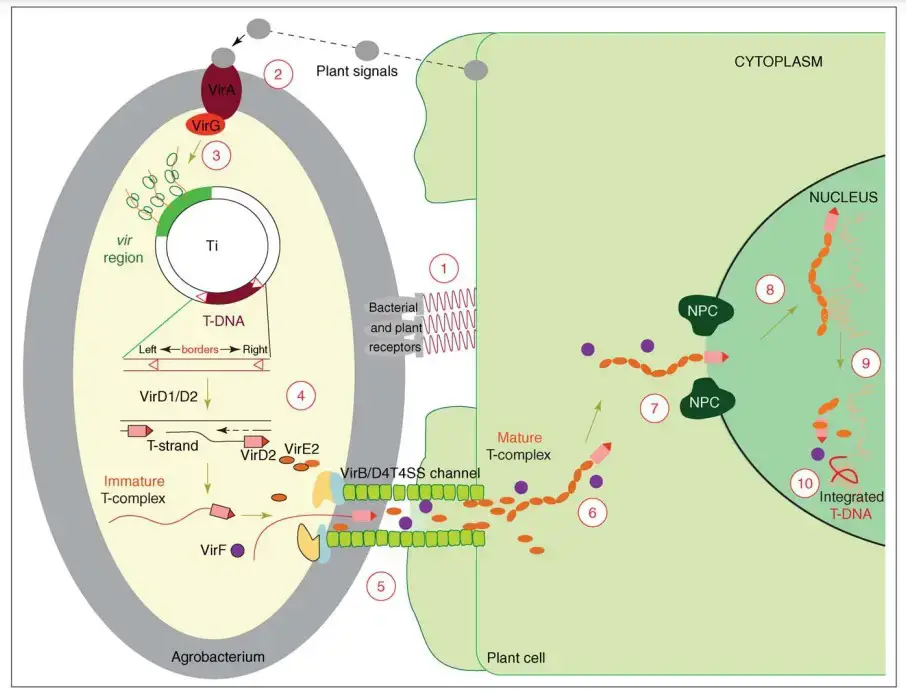
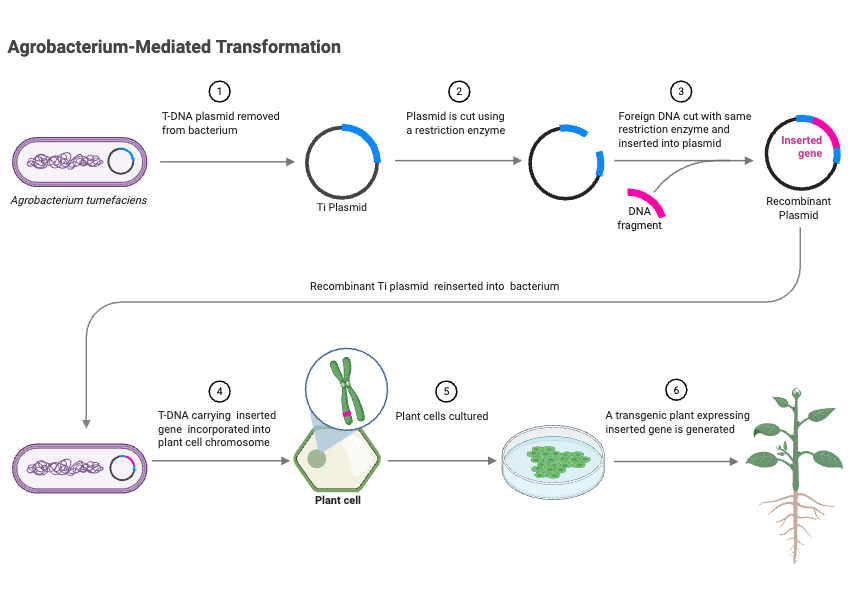
Process of T-DNA transfer and integration
The process of T-DNA transfer and integration into the host plant genome involves several key steps:
- Signal induction to Agrobacterium
Wounded plant cells release phenolic compounds and sugars, such as acetosyringone, which are recognized by Agrobacterium through the VirA receptor. This recognition activates the VirA/VirG two-component system, leading to the expression of virulence (vir) genes on the Ti plasmid. - Attachment of Agrobacterium to plant cells
Agrobacterium attaches to plant cells via polysaccharides, particularly cellulose fibers produced by the bacterium. This attachment is facilitated by the interaction between bacterial surface structures and plant cell wall components. - Production of virulence proteins
Signal induction activates the expression of vir genes, leading to the production of virulence proteins such as VirD1, VirD2, VirE2, and VirB. These proteins are essential for processing and transferring T-DNA into plant cells. - Production of T-DNA strand
The VirD1/D2 complex recognizes the 25 bp border sequences flanking the T-DNA region on the Ti plasmid. VirD2 nicks the DNA at the right border, releasing a single-stranded T-DNA (T-strand) that is covalently bound to VirD2. - Transfer of T-DNA out of Agrobacterium
The T-DNA-VirD2 complex is exported from Agrobacterium through the type IV secretion system (T4SS), which includes the VirB proteins. This system forms a channel through which the T-DNA complex is translocated into the plant cell. - Transfer of T-DNA into plant cells and integration
Once inside the plant cell, the T-DNA-VirD2 complex is coated with VirE2, which protects the T-DNA from nucleases and facilitates its transport to the nucleus. The complex interacts with plant nuclear import machinery, including importin proteins, to enter the nucleus. - Integration into the plant genome
Within the nucleus, the T-DNA integrates into the plant chromosome through a process known as illegitimate recombination. This integration often results in small deletions at the junctions of T-DNA and plant DNA, and may involve DNA repair mechanisms such as non-homologous end joining.
Organization of Ti plasmid
The Ti plasmid in Agrobacterium species, such as A. tumefaciens, is a large, circular, extrachromosomal DNA molecule approximately 200 kb in size. It harbors several distinct regions essential for its role in plant pathogenesis and genetic transformation.
- T-DNA Region
This segment, ranging from 12 to 24 kb depending on the strain, contains genes responsible for auxin (iaaM), cytokinin (ipt), and opine (ocs) biosynthesis. These genes are termed oncogenes due to their role in tumor formation. The T-DNA is flanked by conserved 24 bp border sequences, critical for its excision and transfer into plant cells. - Virulence (vir) Region
Located outside the T-DNA, this region encodes at least nine operons (virA, virG, virB1–11, virC1–2, virD1–2, virE1–2) essential for T-DNA processing and transfer. The VirA/VirG two-component system senses plant-derived signals, activating the vir genes. The VirB proteins form a type IV secretion system facilitating T-DNA translocation into plant cells. - Opine Catabolism Region
This region encodes enzymes for the uptake and metabolism of opines, nitrogen- and carbon-rich compounds produced by transformed plant cells. Agrobacterium utilizes these opines as sole carbon and nitrogen sources, giving it a competitive advantage in the rhizosphere. - Origin of Replication (oriV)
The oriV sequence within the repABC gene cassette is responsible for the plasmid’s replication and stable maintenance within the bacterial cell. The repA, repB, and repC genes, along with the oriV, ensure the Ti plasmid is inherited by daughter cells during bacterial division.
Applications of Agrobacterium-mediated Gene Transfer
Here are some very important applications of Agrobacterium-mediated Gene Transfer
- Crop Improvement -Crop improvement through Agrobacterium-mediated gene transfer involves the introduction of genes that provide resistance to various biotic and abiotic stresses, including insect pests, diseases, and environmental factors. This process serves to enhance both crop yield and stability.
- Nutritional Enhancement – This approach enables the integration of genes that are responsible for the synthesis of essential nutrients, such as beta-carotene in rice, with the objective of mitigating nutritional deficiencies within various populations.
- Pharmaceutical Production -Pharmaceutical Production involves the genetic engineering of plants to synthesise therapeutic proteins, vaccines, and antibodies. This innovative process, referred to as “pharming,” presents a cost-effective alternative to conventional production methods.
- Bioremediation -Bioremediation involves the use of transgenic plants engineered to degrade environmental pollutants, including heavy metals and organic toxins. This process plays a significant role in efforts aimed at environmental cleanup.
- Functional Genomics – Functional genomics is a technique utilised to investigate gene function through the creation of knockout or overexpressing plant lines. This approach facilitates a deeper understanding of the roles that genes play in plant development and responses to stress.
- Industrial Applications – Genetically modified plants are designed to synthesise industrial enzymes, biodegradable plastics, and various bio-based products, thereby contributing to sustainable industrial practices.
- Model Organism Development -Agrobacterium-mediated transformation serves as a pivotal technique in the development of genetically modified model organisms, particularly in the case of Arabidopsis thaliana. This method significantly enhances research capabilities within the fields of plant biology and biotechnology.
- Gene Editing – This technique functions as a foundational platform for CRISPR/Cas9-based gene editing in plants, facilitating accurate alterations to plant genomes for the purposes of research and enhancement of crop varieties.
- Horticultural Advancements -Horticultural advancements involve the application of techniques aimed at developing ornamental plants with modified characteristics, including variations in flower colour and extended shelf life. These modifications are designed to meet the preferences of consumers within the horticulture sector.
- Seedless Crop Development – The process of Agrobacterium-mediated transformation is employed to produce seedless varieties of fruits such as watermelon and grapes, thereby improving consumer appeal and marketability.
Limitations of Agrobacterium-mediated Gene Transfer
- Narrow Host Range – Agrobacterium-mediated gene transfer is limited to certain plant species, primarily dicots; many monocots and recalcitrant species exhibit poor transformation efficiency.
- Low Transformation Efficiency – The process often results in low numbers of transformed cells, necessitating extensive screening and regeneration efforts.
- Regeneration Challenges – Inefficient plant regeneration from transformed tissues hampers the recovery of stable transgenic lines.
- T-DNA Integration Complexity – Unpredictable integration sites and copy numbers can lead to variable expression and potential gene silencing.
- Selectable Marker Concerns – The use of selectable markers, such as antibiotic resistance genes, raises biosafety and regulatory issues.
- Somaclonal Variation – Transformation and tissue culture processes can induce genetic variations, affecting the uniformity of transgenic plants.
- Species-Specific Protocols – Transformation protocols need to be optimized for each plant species, requiring significant time and resources.
- Ethylene Sensitivity – High ethylene production during co-cultivation can inhibit Agrobacterium-mediated transformation.
- Plasmid Size Limitations – Large plasmids may reduce transformation efficiency due to size constraints.
- Regulatory and Public Perception – Concerns over genetically modified organisms (GMOs) can lead to public resistance and stringent regulatory hurdles.
Advantages of Agrobacterium-mediated Gene Transfer
- Efficient Gene Transfer – Agrobacterium-mediated transformation enables the stable integration of transgenes into the plant genome, facilitating the introduction of desired traits such as disease resistance and improved nutritional content.
- Precision and Flexibility – The method allows for precise insertion of genes with fewer rearrangements, offering flexibility in designing constructs with specific promoters and selectable markers.
- Cost-Effectiveness – Compared to other transformation techniques like particle bombardment, Agrobacterium-mediated transformation is relatively low-cost, making it accessible for various research and commercial applications.
- Wide Applicability – This technique is applicable to a broad range of plant species, including many dicots and some monocots, expanding its utility in crop improvement programs.
- Stable Transgene Expression – Agrobacterium-mediated transformation often results in stable transgene expression, ensuring the consistent manifestation of introduced traits across generations.
- Marker-Free Systems – Advancements in marker-free strategies, such as the Cre/lox recombination system, have been developed to eliminate selectable marker genes post-transformation, addressing biosafety concerns.
- Integration with Genome Editing – The method serves as an effective delivery system for CRISPR/Cas9 components, enabling precise genome editing in plants for functional genomics and crop improvement.
- High Transformation Efficiency – Utilizing embryogenic callus as target material has been shown to increase transformation efficiency, providing a reliable source for generating transgenic plants.
- Reduced Metabolic Burden – The binary vector system allows for the expression of only necessary vir genes, minimizing the metabolic load on the host plant during transformation.
- Modular Vector Systems – The use of modular binary vectors facilitates the introduction of multiple genes into a single plant, enhancing the complexity and functionality of transgenic constructs.
FAQ
What is Agrobacterium-mediated gene transfer?
Agrobacterium-mediated gene transfer is a genetic engineering technique used to transfer foreign genes into the genome of a plant cell using a bacterium called Agrobacterium tumefaciens.
How does Agrobacterium-mediated gene transfer work?
Agrobacterium-mediated gene transfer works by using a modified form of Agrobacterium tumefaciens that can transfer foreign DNA into the plant cell. The foreign DNA is incorporated into the genome of the plant cell, resulting in the expression of the transferred gene.
What types of plants can be transformed using Agrobacterium-mediated gene transfer?
Agrobacterium-mediated gene transfer has been successfully used to transform a wide range of plant species, including crops, ornamental plants, and model plants.
What are the advantages of Agrobacterium-mediated gene transfer over other genetic engineering techniques?
Agrobacterium-mediated gene transfer is considered to be a highly efficient and precise method for introducing foreign genes into plants. It also allows for the transfer of larger DNA fragments compared to other methods.
What are the potential applications of Agrobacterium-mediated gene transfer?
Agrobacterium-mediated gene transfer has a wide range of potential applications, including the development of crops with improved traits such as disease resistance, increased yield, and enhanced nutritional value.
Is Agrobacterium-mediated gene transfer safe for the environment?
Agrobacterium-mediated gene transfer has been extensively studied and is considered to be safe for the environment. However, as with any genetic engineering technique, the potential risks associated with the release of genetically modified organisms into the environment must be carefully evaluated.
Can Agrobacterium-mediated gene transfer be used to transfer genes between different species?
Agrobacterium-mediated gene transfer is primarily used to transfer genes between plants of the same or closely related species. However, it is possible to use this technique to transfer genes between more distantly related species.
What are the limitations of Agrobacterium-mediated gene transfer?
Agrobacterium-mediated gene transfer is limited by the ability of the Agrobacterium to infect the target plant species. It is also limited by the availability of suitable plant tissue for transformation.
How long does it take to generate a transgenic plant using Agrobacterium-mediated gene transfer?
The time required to generate a transgenic plant using Agrobacterium-mediated gene transfer can vary depending on the target plant species and the specific gene of interest. However, it typically takes several months to generate a transgenic plant using this technique.
Are there any ethical concerns associated with Agrobacterium-mediated gene transfer?
As with any genetic engineering technique, there may be ethical concerns associated with the use of Agrobacterium-mediated gene transfer, particularly with regard to the potential impact on biodiversity and the use of genetically modified organisms in agriculture. These concerns should be carefully evaluated and addressed before using this technique in a commercial or agricultural context.
- Mehrotra S, Goyal V. Agrobacterium-mediated gene transfer in plants and biosafety considerations. Appl Biochem Biotechnol. 2012 Dec;168(7):1953-75. doi: 10.1007/s12010-012-9910-6. Epub 2012 Oct 23. PMID: 23090683.
- Hwang, H.-H., Yu, M., & Lai, E.-M. (2017). Agrobacterium-Mediated Plant Transformation: Biology and Applications. The Arabidopsis Book, 15, e0186. doi:10.1199/tab.0186
- Amilia Pratiwi, R., & Imam Surya, M. (2020). Agrobacterium-Mediated Transformation. IntechOpen. doi: 10.5772/intechopen.91132
- Gelvin SB. Agrobacterium-mediated plant transformation: the biology behind the “gene-jockeying” tool. Microbiol Mol Biol Rev. 2003 Mar;67(1):16-37, table of contents. doi: 10.1128/MMBR.67.1.16-37.2003. PMID: 12626681; PMCID: PMC150518.
- Tzfira, T., & Citovsky, V. (2006). Agrobacterium-mediated genetic transformation of plants: biology and biotechnology. Current Opinion in Biotechnology, 17(2), 147–154. doi:10.1016/j.copbio.2006.01.009
- Hensel, G., Kastner, C., Oleszczuk, S., Riechen, J., & Kumlehn, J. (2009). Agrobacterium-Mediated Gene Transfer to Cereal Crop Plants: Current Protocols for Barley, Wheat, Triticale, and Maize. International Journal of Plant Genomics, 2009, 1–9. doi:10.1155/2009/835608
- Hohn, B., Koukolíková-Nicola, Z., Bakkeren, G., & Grimsley, N. (1989). Agrobacterium-mediated gene transfer to monocots and dicots. Genome, 31(2), 987–993. doi:10.1139/g89-172
- https://bp.ueb.cas.cz/pdfs/bpl/2009/02/01.pdf
- https://phytopharmajournal.com/assets/pdf_files/Vol11_Issue2_11.pdf
- http://globalsciencebooks.info/Online/GSBOnline/images/0812/TPJ_2(2)/TPJ_2(2)127-137o.pdf
- https://labassociates.com/agrobacterium-mediated-plant-genetic-transformation-an-overview
- https://www.deshbandhucollege.ac.in/pdf/resources/1589512616_Z(H)-VI-Biotech-1.pdf
- https://mgcub.ac.in/pdf/material/202004260029504668c533f4.pdf
- https://goldbio.com/articles/article/a-quick-overview-of-agrobacterium-for-plant-transformation
- https://www.mybiosource.com/learn/testing-procedures/agrobacterium-mediated-gene-transfer/
- https://www.davuniversity.org/images/files/study-material/Agrobacterium-mediated%20gene%20transfer.pdf
- https://www.slideshare.net/NISHANTHSEKAR1/agrobacterium-mediated-gene-transfer-77002058
- https://davuniversity.org/images/files/study-material/Agrobacterium-mediated%20gene%20transfer.pdf
- https://goldbio.com/documents/1018/AgrobacteriumTumefaciens-mediated%20Transformation%20%28AtMtT%29%20of%20Colletotrichum%20graminicola%20Protocol.pdf?srsltid=AfmBOooDyQWLHYz-2yLD1fd790ba0bcEqbBLUsm7O_1SbVe0GcicJ4_i
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.