Sourav Pan
Transcript
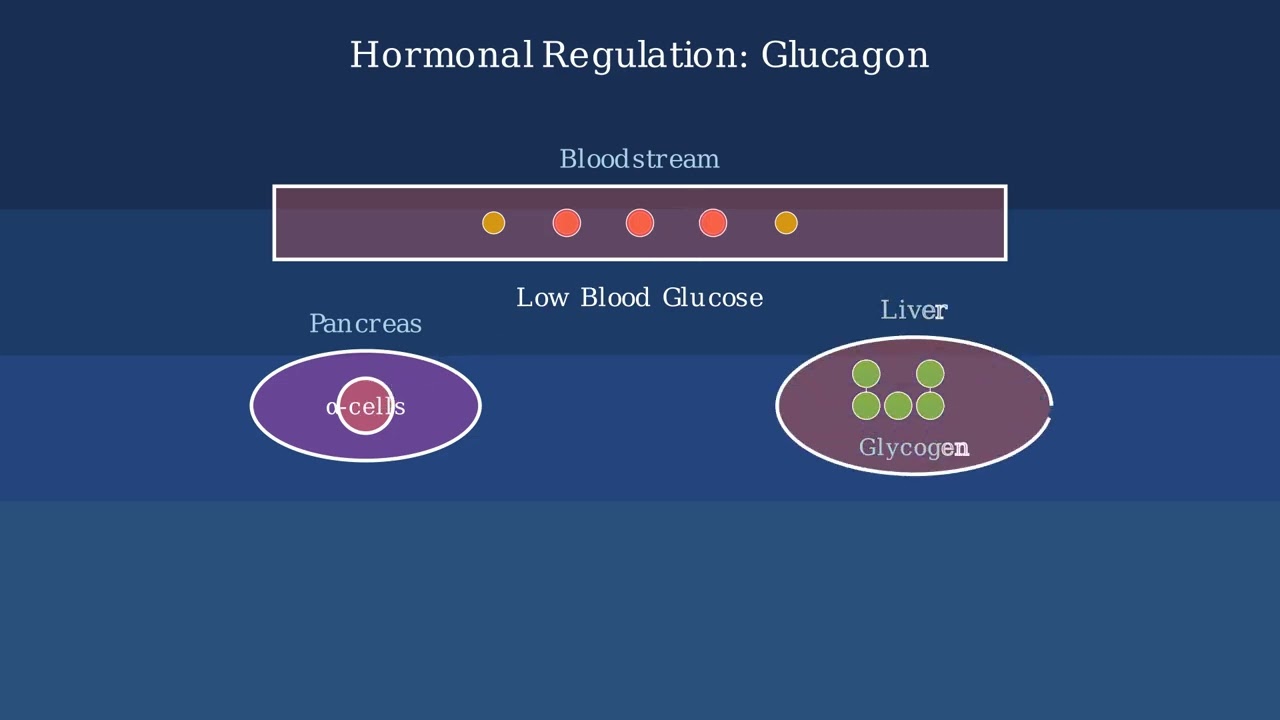
Understanding the key terminology in sterilization is essential for proper infection control.
Sterilization refers to the complete elimination of all microorganisms, including bacteria, fungi, viruses, and spores.
Disinfection eliminates most pathogenic microorganisms, but typically doesn’t affect bacterial spores.
Antiseptics are chemical agents specifically designed for use on living tissues to prevent infection by inhibiting microbial growth.
Asepsis refers to the practices and procedures that prevent contamination from pathogens, creating and maintaining a pathogen-free environment.
These terms are closely related and form a hierarchy of infection control measures.
Asepsis encompasses all techniques for preventing contamination. Within that, disinfection and antiseptics are methods to reduce microbial presence. Sterilization represents the most complete form of microbial elimination.
Let’s examine when each approach is appropriate in different healthcare settings.
Sterilization is essential for critical items entering sterile body tissues, like surgical instruments. Disinfection is suitable for non-critical items that contact intact skin. Antiseptics are used on living tissues requiring microbial reduction. Aseptic techniques create and maintain sterile environments for procedures.
Understanding these key terms is fundamental to infection control practices across all healthcare settings.
Physical sterilization using heat methods is one of the most effective and widely used approaches for eliminating microorganisms.
Heat effectively destroys microorganisms by denaturing proteins and disrupting cell membranes.
Heat sterilization methods can be divided into two main categories: dry heat and moist heat.
Dry heat methods include direct flaming, hot air ovens operating at 160 to 180 degrees Celsius, and incineration. These methods work primarily through oxidation of cell components.
Moist heat methods include boiling at 100 degrees Celsius, steam under pressure, and autoclaving at 121 degrees Celsius for 15 to 20 minutes. These methods work by denaturing and coagulating proteins.
Autoclaving is considered the gold standard for most laboratory equipment sterilization.
An autoclave uses pressurized steam to reach temperatures of 121 degrees Celsius, which is more effective than boiling water.
Autoclaves typically operate at 121 degrees Celsius and 15 pounds per square inch of pressure for 15 to 20 minutes.
Proper loading of an autoclave is crucial for effective sterilization. Items should be arranged with sufficient space between them to allow steam circulation.
Different heat sterilization methods are appropriate for different applications. Autoclaving is ideal for laboratory equipment and media, hot air ovens for glassware and metal instruments, and direct flaming for inoculating loops and needles.
Heat sterilization remains one of the most reliable methods for eliminating microorganisms in laboratory and healthcare settings.
Sunlight acts as a natural sterilization method through its ultraviolet radiation component.
Sunlight contains different types of UV radiation. UVA has minimal germicidal effects, UVB has moderate sterilization capability, while UVC is most effective but is largely filtered by the Earth’s atmosphere.
UV radiation damages microbial DNA by creating thymine dimers, which prevent proper DNA replication. This disruption leads to cell death or inability to reproduce.
Solar disinfection has several practical applications. SODIS is a water purification method where contaminated water in clear plastic bottles is exposed to sunlight. Sunlight is also used for disinfecting laundry and surfaces in resource-limited settings.
Despite its benefits, solar disinfection has limitations. It’s highly dependent on weather conditions and cloud cover. Effective sterilization requires at least six hours of exposure, and sunlight cannot penetrate opaque materials.
Ozone, a triatomic oxygen molecule with the formula O₃, is a powerful oxidizing agent used for sterilization.
Ozone’s sterilizing effect comes from its ability to oxidize cellular components. It damages cell membranes and proteins, disrupts enzymatic systems, and breaks down nucleic acids, ultimately leading to microbial death.
Ozone is widely used in water treatment systems to disinfect drinking water and wastewater. It’s employed in air purification systems to eliminate odors and microorganisms. Ozone also finds applications in food processing and medical equipment sterilization.
Ozone has several limitations. It can be harmful to humans at high concentrations, requiring careful monitoring and containment. It has a short half-life, meaning it must be generated on-site. Ozone can also damage certain materials like rubber and plastics.
Both sunlight and ozone offer effective physical sterilization methods with specific applications and limitations that must be considered when implementing them in practical settings.
Study Materials
No study materials available for this video.
Helpful: 0%
Related Videos