Sourav Pan
Transcript
Section 1: What is Agrobacterium-mediated Gene Transfer?
Agrobacterium-mediated gene transfer is a biotechnological technique that uses soil bacteria to insert foreign genes into plant cells.
This method has become one of the most widely used approaches for creating genetically modified plants.
The technique uses Agrobacterium tumefaciens, a soil bacterium that naturally infects plant wound sites.
What makes this bacterium special is its natural ability to transfer a segment of DNA, called T-DNA, from its tumor-inducing plasmid into the plant’s genome.
Let’s explore how this process works and why it’s so important for plant biotechnology.
To understand Agrobacterium-mediated gene transfer, we need to compare the natural process with its biotechnological application.
In nature, Agrobacterium transfers T-DNA containing genes that alter plant hormone production, causing tumorous growths known as crown gall disease.
Scientists have ingeniously modified this system by replacing the tumor-inducing genes with genes of interest, creating a powerful tool for plant genetic engineering.
Agrobacterium is a soil-dwelling bacterium that has evolved a remarkable ability to genetically modify plants.
In nature, Agrobacterium targets plant wound sites, exploiting the plant’s vulnerability to infection.
The bacteria are attracted to chemical signals released by wounded plant tissue.
Once at the wound site, Agrobacterium transfers a portion of its DNA into the plant cells, causing crown gall disease – characterized by tumor-like growths.
The transferred genes force the plant to produce unique compounds called opines. These serve as specialized nutrients that only the Agrobacterium can use, creating a parasitic relationship.
Scientists recognized the remarkable potential of Agrobacterium’s natural gene transfer ability and modified it for biotechnology applications.
By removing the disease-causing genes while preserving the DNA transfer mechanism, researchers transformed this plant pathogen into a powerful tool for genetic engineering.
This ingenious repurposing allows scientists to insert beneficial genes into plant genomes with precision, without causing disease. Essentially, nature’s genetic engineer has been harnessed for sustainable crop improvement.
Agrobacterium is a genus of soil bacteria widely used for plant genetic transformation.
There are three main Agrobacterium species used in plant biotechnology, each with unique characteristics.
Agrobacterium tumefaciens is the most commonly used species. It causes crown gall disease in plants, contains the Ti plasmid, and can infect a wide range of plant species.
Agrobacterium rhizogenes induces hairy root disease. It contains the root-inducing or Ri plasmid and is particularly useful for generating transgenic root cultures.
Agrobacterium vitis is specific to grapevines, causing grape crown gall disease. It has a narrower host range and is primarily used in viticulture applications.
Let’s explore how each species is applied in plant biotechnology.
Agrobacterium tumefaciens is widely used for crop improvement, including introducing herbicide resistance, insect resistance, and enhancing nutritional qualities.
Agrobacterium rhizogenes is particularly valuable for producing secondary metabolites and medicinal compounds, studying root systems, and developing stress-tolerant plants.
Agrobacterium vitis is applied specifically in grapevine improvement, enhancing disease resistance, wine quality, and drought tolerance in vineyards.
When choosing an Agrobacterium species for plant transformation, consider these guidelines:
Choose Agrobacterium tumefaciens for most dicot crops and general applications. Use Agrobacterium rhizogenes when root development or root cultures are desired. Select Agrobacterium vitis specifically for grapevine transformation. Always consider bacterial strain compatibility with your plant host, and match your transformation goals with the specific plasmid properties of each species.
Each Agrobacterium species offers unique advantages for specific plant transformation objectives. Understanding their differences is crucial for successful genetic engineering in plants.
For successful Agrobacterium-mediated transformation, specific laboratory equipment is essential.
To summarize, proper equipment and maintaining sterile conditions are critical for successful Agrobacterium-mediated transformation.
This section covers the essential materials and reagents required for Agrobacterium-mediated gene transfer.
Plant tissue culture media forms the foundation for growing plant tissues. Common formulations include Murashige and Skoog medium and Gamborg’s B5 medium, which provide essential macro and micronutrients, vitamins, and carbon sources for plant growth.
Selection antibiotics are crucial for identifying transformed plant cells. Antibiotics like kanamycin and hygromycin select for plant cells containing the resistance gene, while carbenicillin and cefotaxime help eliminate residual Agrobacterium after transformation.
Plant growth regulators control cell division and differentiation. Auxins like 2,4-D and NAA promote callus formation and root development, while cytokinins such as BAP and zeatin stimulate shoot development. The balance between these hormones determines regeneration outcomes.
Sterile culture containers are essential for maintaining aseptic conditions. Petri dishes, culture tubes, and specialized plant culture vessels provide the environment for plant growth. Proper sealing with micropore tape or Parafilm ensures sterility while allowing gas exchange.
Bacterial culture media are used for growing Agrobacterium strains. LB, YEB, and MG/L media provide nutrients for bacterial growth. These media are supplemented with antibiotics to maintain the selection pressure for the binary vector system.
Quality and sterility are non-negotiable in Agrobacterium-mediated transformation. Contamination with fungi or bacteria can lead to complete experimental failure. All media must be prepared with high-grade reagents and properly sterilized by autoclaving or filter sterilization for heat-sensitive components.
To ensure successful Agrobacterium-mediated transformation, maintain a carefully prepared inventory of all essential materials including media, antibiotics, growth regulators, sterile containers, and bacterial culture supplies.
With these materials prepared, we are ready to proceed to the next steps in the transformation process.
Preparing Agrobacterium cultures is a critical step for successful plant transformation.
First, select a single colony of Agrobacterium containing your gene of interest. Inoculate it into liquid media such as LB or YEB, supplemented with appropriate antibiotics for selection.
Incubate the inoculated culture at 28 degrees Celsius with shaking at 180 to 200 RPM. Monitor bacterial growth by measuring optical density at 600 nanometers. The optimal density for transformation is between 0.6 and 0.8, which typically occurs during the log phase of bacterial growth.
Once the culture reaches optimal density, transfer it to sterile centrifuge tubes. Centrifuge at 3000 to 5000g for 10 minutes to pellet the bacterial cells. After centrifugation, carefully discard the supernatant, leaving the bacterial pellet intact.
Resuspend the bacterial pellet in fresh liquid medium. Adjust the concentration to OD600 of 0.3 to 0.5 for optimal transformation efficiency. Add acetosyringone to induce virulence genes. Allow the suspension to incubate for 1 to 3 hours before using it to infect plant tissue.
To maximize transformation efficiency, use fresh bacterial cultures that are 18 to 24 hours old. Ensure precise optical density measurements and maintain sterile conditions throughout the process. For optimal results, adjust the protocol based on the specific plant species being transformed.
Explant preparation is a critical step in Agrobacterium-mediated transformation.
Explants are plant tissue segments that are used for transformation and the regeneration of transgenic plants.
Different types of explants can be used depending on the plant species and experimental goals. Common explant types include leaf discs, cotyledons, hypocotyls, and stem segments.
Explants must be carefully prepared to create wound sites. For leaf discs, a cork borer is commonly used to cut uniform circles from intact leaves.
Wounding is essential for successful transformation. Cut edges release phenolic compounds that attract Agrobacterium and create entry points for bacterial invasion.
The choice of explant varies significantly by plant species. Some plants, like tobacco, transform efficiently using leaf discs, while others may require specialized tissue like embryogenic callus.
Multiple factors affect transformation success rates. The age of donor tissue and size of explants are particularly important, with young tissues and smaller explants generally yielding better results.
When preparing explants, maintain strict sterile conditions to prevent contamination. Use young, actively growing tissue and create clean, precise cuts to optimize transformation efficiency.
Proper explant preparation is a foundation for successful Agrobacterium-mediated transformation and can significantly impact your experimental outcomes.
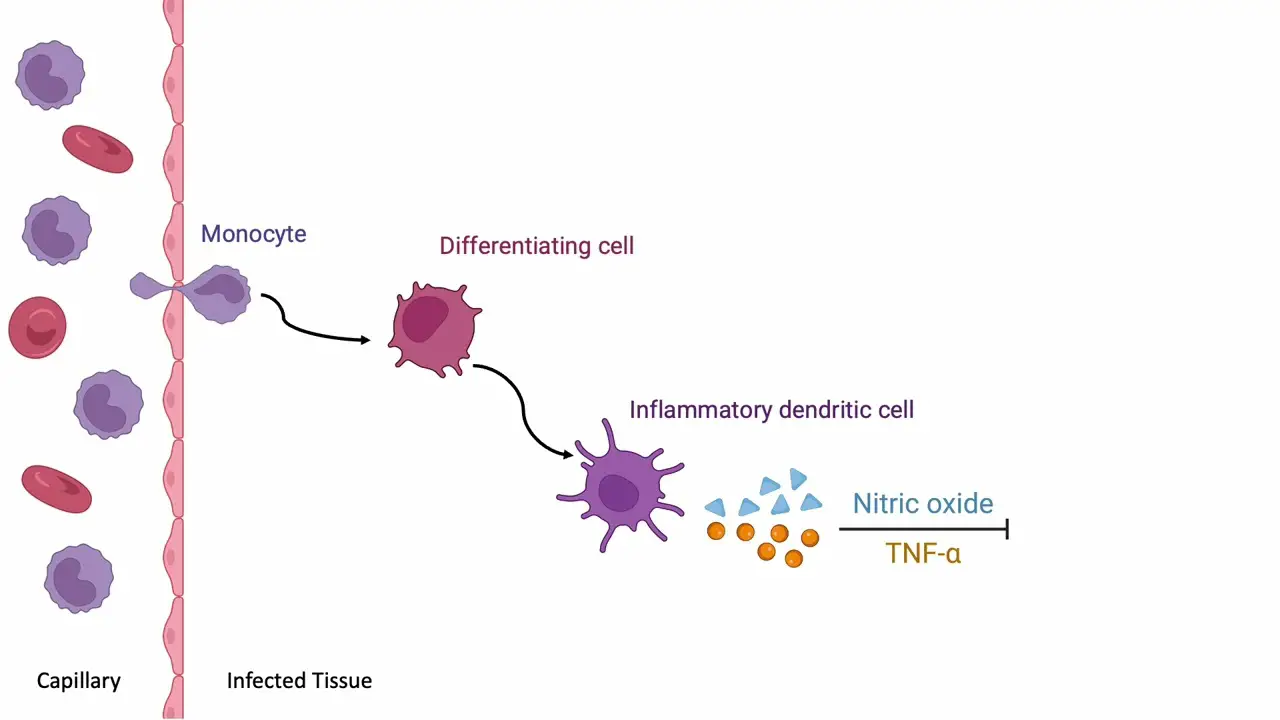
Section 11: Infection and Co-cultivation
The infection process begins by immersing plant explants in an Agrobacterium suspension.
The bacteria surround and attach to the plant tissue, initiating the gene transfer process.
After infection, the explants are transferred to co-cultivation media without any selection agents.
Co-cultivation typically lasts for two to three days. During this critical period, Agrobacterium transfers its T-DNA into the plant cells.
At the molecular level, Agrobacterium attaches to the plant cell and creates a channel to transfer its T-DNA.
Controlling co-cultivation conditions is crucial for successful transformation. Important factors include temperature, light, pH, and hormone balance.
It’s essential to balance transformation efficiency with bacterial control. Extended co-cultivation can lead to bacterial overgrowth, reducing transformation success.
After co-cultivation of plant tissue with Agrobacterium, washing is a critical step.
Excess bacteria must be removed to prevent overgrowth that could damage or kill the plant tissue.
Proper washing protects the explant’s viability while ensuring that transformation has had sufficient time to occur.
Let’s examine the step-by-step washing procedure to eliminate excess Agrobacterium from plant tissues.
First, prepare sterile wash solutions and antibiotics at appropriate concentrations.
Next, carefully transfer the co-cultivated explants to a sterile container to begin washing.
Wash the explants three to four times with sterile distilled water to remove the majority of bacteria.
In the final wash, add appropriate antibiotics to kill any remaining bacteria.
Finally, transfer the explants to selection media containing antibiotics to continue the transformation process.
Several antibiotics can be used to eliminate Agrobacterium. Here are the most commonly used options and their typical concentrations.
Cefotaxime is commonly used at concentrations between 250 and 500 milligrams per liter to inhibit bacterial cell wall synthesis.
Timentin, a mixture of ticarcillin and clavulanic acid, provides broad-spectrum activity at 150 to 300 milligrams per liter.
Carbenicllin serves as an alternative to cefotaxime and is used at similar concentrations.
Now, let’s visualize how the bacterial washing process works.
Initially, the explant is surrounded by a high density of Agrobacterium cells after co-cultivation.
After the first wash with sterile water, we see a significant reduction in bacterial numbers.
The second wash further reduces bacterial density.
Finally, after treatment with antibiotics, virtually all bacteria are eliminated while the transformed plant DNA remains integrated.
There are several critical considerations for successful bacterial elimination.
Timing is crucial. Allow 24 to 48 hours of co-cultivation before washing to ensure proper gene transfer.
Wash solutions should be at room temperature to minimize stress to the plant tissues.
Gentle handling of explants is essential to minimize tissue damage during the washing process.
Maintain strict aseptic conditions throughout to prevent contamination.
Select antibiotics based on the specific Agrobacterium strain used for transformation.
After plant tissues have been infected with Agrobacterium and co-cultivated, the next critical step is selecting cells that have successfully incorporated the transferred DNA.
The selection process relies on antibiotic or herbicide resistance genes that are transferred alongside the genes of interest. These resistance genes allow transformed cells to survive in the presence of selection agents.
When selection agents are added to the culture medium, non-transformed cells cannot survive because they lack the resistance gene.
In contrast, transformed cells express the resistance gene and can detoxify or block the selection agent, allowing them to grow and divide.
Several selection agents are commonly used in plant transformation. Each works with a specific resistance gene that counteracts the agent’s toxic effects.
The mechanism of resistance varies depending on the selection system used. In most cases, the resistance gene encodes an enzyme that inactivates the antibiotic or herbicide.
The selection process isn’t a one-time event. Selection pressure must be maintained through multiple subcultures over several weeks to ensure only truly transformed cells continue to grow.
With each subculture, the proportion of transformed cells increases as any remaining non-transformed cells are eliminated. This process typically takes three to four weeks.
To summarize the key points about selection: Selection agents eliminate non-transformed cells while transformed cells with resistance genes survive. Multiple subcultures ensure pure transformants, and selection pressure must be maintained throughout the process.
Root induction is a critical phase in plant transformation where regenerated shoots develop root systems to become complete plantlets.
During this phase, regenerated shoots are transferred from shoot induction media to specialized rooting media. This transfer must be done under aseptic conditions to prevent contamination.
Auxins are the key plant hormones responsible for root development. Common auxins used include IAA, NAA, and IBA, which stimulate cell division and differentiation in the stem base.
When exposed to the correct auxin concentration, cells at the base of the shoot begin to divide and differentiate into root primordia, eventually forming a complete root system.
The efficiency of root induction varies greatly between plant species. Some plants, like tobacco or tomato, develop roots easily with standard protocols.
Other species, particularly woody plants and some cereals, are more recalcitrant to rooting and may require higher auxin concentrations or additional treatments to stimulate root development.
For difficult-to-root species, several additional treatments may be employed. These include pulse treatments with high auxin concentrations, the addition of phenolic compounds, or physical wounding of the stem base to stimulate rooting.
Other factors that can enhance rooting include optimizing light and dark exposure periods, adjusting temperature, or adding specific rooting co-factors depending on the plant species.
The end result of successful root induction is a complete plantlet with a functional root system connected to the shoot. These roots will allow the plant to absorb water and nutrients independently.
A well-rooted plantlet represents the final stage before acclimatization to soil conditions, bridging the gap between in vitro and in vivo environments.
After successfully inducing roots, the complete plantlets are ready for the next critical phase: acclimatization to soil conditions.
Acclimatization to soil is a critical step in transferring transformed plantlets from tissue culture to normal growing conditions.
The process begins with carefully removing plantlets from their sterile culture vessels.
These plantlets must be gradually adapted to soil conditions, as they’ve been growing in a protected, high-humidity environment.
The acclimatization process consists of several critical steps:
First, carefully transfer plantlets to a sterile potting mix with good drainage.
Second, cover the plants with a humidity dome or plastic bag to maintain high humidity.
Third, gradually reduce humidity by incrementally opening vents or lifting the dome over one to two weeks.
Fourth, provide controlled light exposure, starting with indirect light and slowly increasing intensity.
Finally, transition to a normal fertilizer regime as plants establish.
The most critical aspect of acclimatization is the gradual humidity reduction.
Initially, plants are kept at nearly one hundred percent humidity using a dome or plastic covering.
After about a week, begin creating small openings to gradually reduce humidity.
By the end of the second week, the cover can be removed completely as plants adapt to ambient humidity.
Several key factors determine successful acclimatization of transformed plantlets:
First, timing is critical – transfer plants during their active growth phase.
Temperature should be maintained between twenty-two and twenty-five degrees Celsius.
Light exposure should start with indirect light and increase gradually.
The substrate should be a well-draining sterile mix, often containing perlite.
Regular monitoring is essential to identify and address any signs of stress.
Proper acclimatization ensures plants can successfully transition from controlled lab conditions to natural environments.
During this process, plants shift from heterotrophic to fully autotrophic growth.
Functional stomata develop for normal gas exchange.
Plants form a protective cuticle layer to prevent water loss.
The root system strengthens for efficient nutrient uptake from soil.
All these adaptations enable the transformed plants to establish successfully in natural growing conditions.
After the plant transformation process, confirming successful gene integration is a critical step.
Scientists use four key methods to verify that the genetic transformation was successful.
PCR, or Polymerase Chain Reaction, detects the presence of the inserted gene in the plant DNA.
This technique uses specific primers that bind to and amplify the transgene sequence if it’s present, providing a quick and reliable first confirmation.
RT-PCR, or Reverse Transcription PCR, goes beyond detection to verify if the transgene is being expressed at the RNA level.
This method first converts messenger RNA to complementary DNA, then performs PCR, indicating whether the gene is functionally active in the plant.
Southern blotting is a more definitive technique that confirms actual integration of the gene into the plant genome.
It can also determine the number of gene copies inserted, though it’s more labor-intensive than PCR-based methods.
Phenotypic analysis involves observing visible traits resulting from transgene expression, such as herbicide resistance or color changes.
While it provides simple visual confirmation, not all transgenes produce visible phenotypes, so molecular methods are still essential.
Molecular verification before proceeding with further research is absolutely critical.
It prevents false positives, saves resources, and provides the validation required for publication and regulatory approval.
Environmental conditions significantly impact both transformation efficiency and regeneration success in Agrobacterium-mediated gene transfer.
Temperature is a critical factor. The optimal range is twenty-two to twenty-five degrees Celsius. Higher temperatures promote bacterial overgrowth, while lower temperatures reduce virulence gene expression.
Light conditions also play a key role. The standard sixteen hour light, eight hour dark cycle with moderate intensity provides optimal results. Higher light intensity can induce oxidative stress.
Humidity should be maintained at sixty to seventy percent. Proper air flow and carbon dioxide levels also influence transformation success by affecting plant physiology.
To summarize, the optimal environmental conditions include temperatures of twenty-two to twenty-five degrees Celsius, sixteen hour light and eight hour dark cycle, light intensity of fifty to one hundred micromoles per square meter per second, and relative humidity of sixty to seventy percent.
Deviations from optimal conditions can significantly reduce transformation efficiency. Temperature stress decreases T-DNA transfer, light stress causes oxidative damage, and humidity fluctuations lead to tissue desiccation.
For best results, use controlled growth chambers to maintain consistent conditions. Monitor parameters throughout the transformation process and adjust protocols based on your specific plant species requirements.
Agrobacterium-mediated genetic transformation has revolutionized crop improvement by enabling precise insertion of beneficial genes into plant genomes.
Crop improvement through genetic engineering focuses on four key trait categories that address major agricultural challenges.
The impact of genetically engineered crops extends beyond individual traits, leading to increased yields, reduced chemical use, enhanced food security, and improved nutritional outcomes.
Bt cotton contains genes from Bacillus thuringiensis bacteria that produce proteins toxic to specific insect pests like bollworm. This technology has reduced insecticide use by up to 80 percent, increased yields by 20 to 30 percent, and has been adopted in more than 15 countries worldwide.
Herbicide-tolerant crops contain genes that allow them to survive application of specific herbicides that would normally kill the crop along with weeds. Popular examples include Roundup Ready soybeans with glyphosate resistance and Liberty Link corn with glufosinate tolerance, both using bacterial genes to confer resistance.
Drought-tolerant crops use genes that regulate stress response pathways to improve water use efficiency and yield stability. Commercial examples include DROUGHTGARD maize, which maintains yields with 30 to 50 percent less water, and experimental drought-tolerant wheat varieties being developed for arid regions.
Golden Rice is engineered to contain beta-carotene, a precursor of vitamin A, in the rice endosperm. It contains genes from soil bacteria and either daffodil or maize to enable this biosynthesis pathway. This innovation has the potential to prevent 250,000 to 500,000 cases of childhood blindness annually in regions where vitamin A deficiency is prevalent.
While Agrobacterium-mediated transformation is a powerful technique, it faces several limitations and challenges that researchers must overcome.
Study Materials
Agrobacterium-Mediated Gene Transfer in Plants - Protocolo
Agrobacterium-Mediated Gene Transfer - Protocol, Applications, Advantages
Helpful: 100%
Related Videos