What is a Telomere?
Telomeres are crucial components found at the ends of chromosomes. They are made up of repetitive nucleotide sequences that protect the genetic material from damage. Each time a cell divides, the telomeres shorten, playing a key role in cellular aging and health. Here’s a breakdown of what telomeres are, how they work, and why they matter.
- What They Are
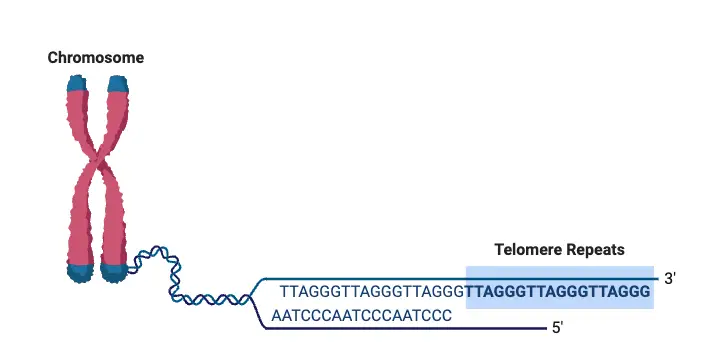
- Telomeres are repetitive DNA sequences located at the ends of chromosomes.
- In humans, the repeated sequence is 5′−TTAGGG−3′, and it repeats around 3,000 times, which can span up to 15,000 base pairs.
- They don’t code for proteins but play a crucial role in maintaining chromosome integrity.
- Structure
- Telomeres are made up of non-coding regions, meaning they don’t produce proteins.
- These sequences are rich in guanine nucleotides, making them critical for their function.
- At the very end of each telomere is a single-stranded overhang that forms a loop called the T-loop, which protects the chromosome from being recognized as damaged.
- Function
- Protection: Telomeres act as a shield, preventing the chromosome ends from fraying or sticking together, which could lead to instability.
- Limiting Cell Division: Every time a cell divides, the telomeres shorten slightly. This is a built-in limit to how many times a cell can divide, ensuring that the cell doesn’t divide infinitely.
- Telomerase: In certain cells like stem or cancer cells, an enzyme called telomerase can extend the telomeres, allowing these cells to bypass the normal aging process and keep dividing indefinitely.
- Impact on Aging and Disease
- Telomere shortening is linked to aging and age-related diseases. As cells divide, telomeres naturally get shorter, which contributes to the overall aging of tissues.
- On the flip side, some cells—like cancer cells—often have higher levels of telomerase activity, allowing them to keep dividing uncontrollably. This can be a key factor in tumor growth.
Telomeres are essential in maintaining the stability of our genetic material, but they also play a delicate balancing act in aging and disease prevention.
What is Telomerase?
Telomerase is an enzyme that helps keep the telomeres—the protective caps at the ends of chromosomes—at a functional length. It does this by adding specific nucleotide sequences to the telomeres, counteracting the natural shortening that occurs during cell division. Here’s how it works and why it matters:
- What It Is
- How It Works
- Telomerase prevents telomere shortening by adding repetitive sequences to the ends of chromosomes.
- Each time a cell divides, a small part of the telomere is lost. Telomerase adds the TTAGGG sequence to prevent this loss and keep the telomeres intact.
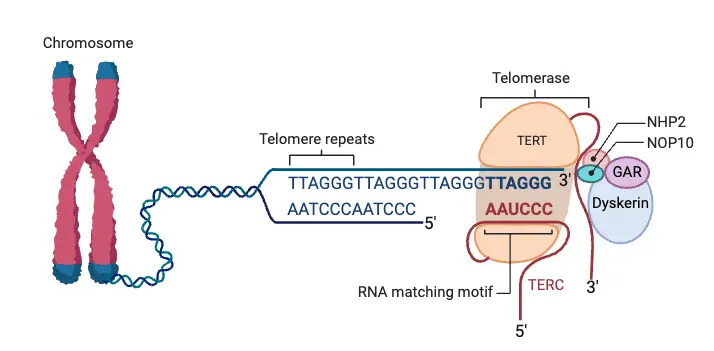
- It works by binding to the single-stranded overhang at the end of a telomere, using its RNA template to extend the telomere length.
- Key Functions
- Length Maintenance: It adds guanine-rich sequences, which are crucial for preserving the chromosome’s integrity during division.
- Cellular Longevity: By extending the telomeres, telomerase ensures that certain cells—like stem cells and germ cells—can continue dividing without entering senescence or apoptosis (programmed cell death).
- Cancer Role: Many cancer cells have reactivated telomerase, allowing them to bypass normal aging processes and continue dividing uncontrollably.
- Clinical Implications
- Aging: As telomeres shorten over time, they are linked to cellular aging and age-related diseases. Telomerase research could provide insights into potential treatments for slowing aging.
- Cancer Treatment: Since cancer cells rely on active telomerase for unrestrained growth, targeting this enzyme is a promising strategy for cancer therapies aimed at limiting tumor growth.
Difference between Telomere and Telomerase
Telomeres and telomerase are both critical components in maintaining cellular stability, but they play distinct roles in the processes of cell division and aging. Here’s a breakdown of their differences:
- What They Are
- Telomeres are repetitive DNA sequences found at the ends of chromosomes.
- Telomerase is an enzyme made up of both protein and RNA, responsible for extending the length of telomeres.
- Composition
- Telomeres consist of non-coding DNA, specifically the repetitive sequence TTAGGG in humans.
- Telomerase consists of a protein component (TERT) and an RNA component (TERC), both of which work together to extend telomeres.
- Function
- Telomeres protect the ends of chromosomes from degradation and prevent them from fusing with other chromosomes during cell division.
- Telomerase actively adds nucleotide repeats to the telomeres to counteract their shortening during cell division, helping maintain chromosome stability.
- Location
- Telomeres are located at the ends of chromosomes in eukaryotic cells.
- Telomerase operates primarily in the nucleus of cells, particularly active in certain cell types like germ cells, stem cells, and cancer cells.
- Role in Aging
- Telomeres shorten each time a cell divides, contributing to cellular aging and the eventual entry of cells into senescence or apoptosis.
- Telomerase helps prevent telomere shortening, allowing some cells, like stem cells, to divide indefinitely and maintain their function.
- Activity in Cells
- Telomeres are present in most somatic cells but shorten over time.
- Telomerase is highly active in germ cells, stem cells, and many cancer cells, but it is inactive in most somatic cells.
- Biological Clock
- Telomeres act as a biological clock that limits the number of times a cell can divide by shortening over time.
- Telomerase resets this clock by adding nucleotide repeats to the telomeres, allowing continued cell division.
- Mechanism of Action
- Telomeres function passively as protective structures during DNA replication and cell division.
- Telomerase functions actively by using its RNA template to extend telomeres, preventing their degradation.
- Clinical Implications
- Telomere shortening is linked to aging and age-related diseases like cardiovascular disease and cancer.
- Telomerase reactivation is commonly found in cancer cells, enabling them to evade normal cellular aging and continue proliferating uncontrollably.
- Therapeutic Potential
- Telomere shortening could be targeted for therapies focused on anti-aging or age-related diseases.
- Telomerase inhibition holds potential as a cancer treatment, aiming to limit tumor growth by preventing the enzyme from extending telomeres.
- Impact on Cancer
- Shortened telomeres lead to chromosomal instability, often a precursor to cancer progression.
- Telomerase is reactivated in many cancers, enabling tumor cells to bypass senescence and divide endlessly.
| Aspect | Telomere | Telomerase |
|---|---|---|
| Definition | Repetitive DNA sequences at the ends of chromosomes. | Enzyme that synthesizes telomere DNA sequences. |
| Composition | Non-coding DNA, specifically the sequence TTAGGG in humans. | Protein (TERT) and RNA (TERC) components. |
| Function | Protects chromosome ends from degradation and prevents fusion with other chromosomes. | Adds nucleotide repeats to telomeres, maintaining their length during cell division. |
| Structure | Tandem repeats of DNA forming a protective cap. | Ribonucleoprotein structure, integrating both RNA and protein elements. |
| Location | Found at the ends of linear chromosomes in eukaryotes. | Found in the nucleus, active in specific cell types. |
| Role in Aging | Shortens with each cell division, contributing to cellular aging and senescence. | Counteracts telomere shortening, allowing certain cells to divide indefinitely. |
| Activity in Cells | Present in most somatic cells but shortens over time. | Highly active in germ cells, stem cells, and many cancer cells; inactive in somatic cells. |
| Biological Clock | Acts as a biological clock, limiting the number of divisions a cell can undergo. | Resets the biological clock by extending telomeres, allowing continued cell division. |
| Genetic Stability | Maintains genetic stability by preventing chromosome end degradation or fusion. | Ensures telomeres remain intact and functional, maintaining genetic stability. |
| Mechanism of Action | Functions passively as a protective structure during DNA replication and division. | Actively adds DNA sequences to telomeres using its RNA template during cell division. |
| Clinical Implications | Shortening is associated with age-related diseases and cellular senescence. | Reactivation is observed in cancer cells, contributing to uncontrolled growth. |
| Research Focus | Studied for its role in aging, longevity, and age-related diseases like cancer. | Studied for therapeutic targets in cancer treatment and anti-aging interventions. |
| Regulation | Length regulated by natural aging and stressors like oxidative stress. | Activity regulated by signaling pathways, gene expression, and environmental cues. |
| Role in Development | Essential for chromosome function during development; ensures genomic stability. | Critical for stem cells to maintain pluripotency and differentiation ability. |
| Presence in Organisms | Present in almost all eukaryotic organisms, including plants, animals, and fungi. | Found in specific cell types (germ cells, stem cells), low or absent in most somatic cells. |
| Telomere Length Variability | Length varies across cell types and species; shorter in somatic cells. | Activity varies across tissues; higher activity correlates with longer telomeres. |
| Evolutionary Role | Evolved to protect genetic material from degradation. | Evolved to extend the lifespan of critical cell types for reproduction and tissue maintenance. |
| Interaction with Other Proteins | Interacts with shelterin complex proteins that protect telomeres. | Interacts with regulatory proteins that modulate its activity and recruitment. |
| Impact on Cancer Biology | Shortened telomeres lead to chromosomal instability, a hallmark of cancer. | Reactivated telomerase allows cancer cells to evade senescence and proliferate. |
| Therapeutic Potential | Targeting telomere shortening could lead to therapies for age-related diseases. | Inhibiting telomerase activity offers a potential strategy for cancer treatment. |
- https://pubmed.ncbi.nlm.nih.gov/15065663/
- https://www.khanacademy.org/science/biology/dna-as-the-genetic-material/dna-replication/a/telomeres-telomerase
- https://www.genome.gov/genetics-glossary/Telomere
- https://testbook.com/key-differences/difference-between-telomere-and-telomerase
- https://pmc.ncbi.nlm.nih.gov/articles/PMC1693310/
- https://www.nature.com/articles/6603671
- https://febs.onlinelibrary.wiley.com/doi/10.1016/j.febslet.2010.05.026
- https://study.com/academy/lesson/telomeres-definition-function-quiz.html
- https://study.com/academy/lesson/what-is-telomerase-definition-function-structure.html
- https://byjus.com/neet/telomeres/
- https://www.britannica.com/science/telomerase
- https://pubmed.ncbi.nlm.nih.gov/21417995/
- https://byjus.com/biology/difference-between-telomere-and-telomerase/