What is BOD?
- BOD is the biochemical oxygen demand, which quantifies the amount of dissolved oxygen (DO) required by aerobic organisms to decompose organic material in a given water sample at a certain temperature and time.
- As BOD is a biological process, it is not a quantitatively precise test. However, BOD is a commonly employed test method that indicates the organic quality of water.
- The BOD is calculated by incubating a sealed sample of water for five days and measuring the oxygen loss since the commencement of the test.
- Two measurements are required for the BOD calculation of a sample. The initial DO is followed by the final DO five days later.
- BOD is measured in milligrammes of oxygen used per litre of sample during five days of incubation at 20 degrees Celsius (BOD5). The BOD has a direct impact on the DO of rivers and streams.
- Leaves, woody debris, topsoil, animal dung, food processing plants, sewage treatment plants, feedlots, malfunctioning septic systems, urban stormwater runoff, and effluents from pulp and paper mills are the sources of BOD.
- The rate of oxygen consumption relies on the water’s temperature, pH, the type of bacteria present, and the type of organic matter present.
- The larger the BOD in a given body of water, the less oxygen is available to aquatic organisms in that body of water.
- Due to increased BOD, aquatic organisms would experience stress, suffocate, and finally perish.
What is COD?
- The chemical oxygen demand (COD) estimates the quantity of DO necessary for the breakdown of organic materials and the oxidation of inorganic compounds such as ammonia and nitrite.
- Commonly, COD tests are performed on sewage or natural water samples that are contaminated with domestic and industrial pollutants.
- A closed water sample is incubated with a strong oxidant, such as potassium dichromate (K2Cr2O7), in conjunction with boiling sulfuric acid (H2SO4) at a certain temperature and for a specific time.
- COD is associated with BOD. COD, however, is the only method for measuring industrial pollutants in water, which cannot be quantified using BOD.
- COD is the only method for measuring cellulose in water. COD is measured by plants that treat wastewater from commercial activity.
Difference between Biochemical Oxygen Demand (BOD) and Chemical Oxygen Demand (COD) – BOD vs COD
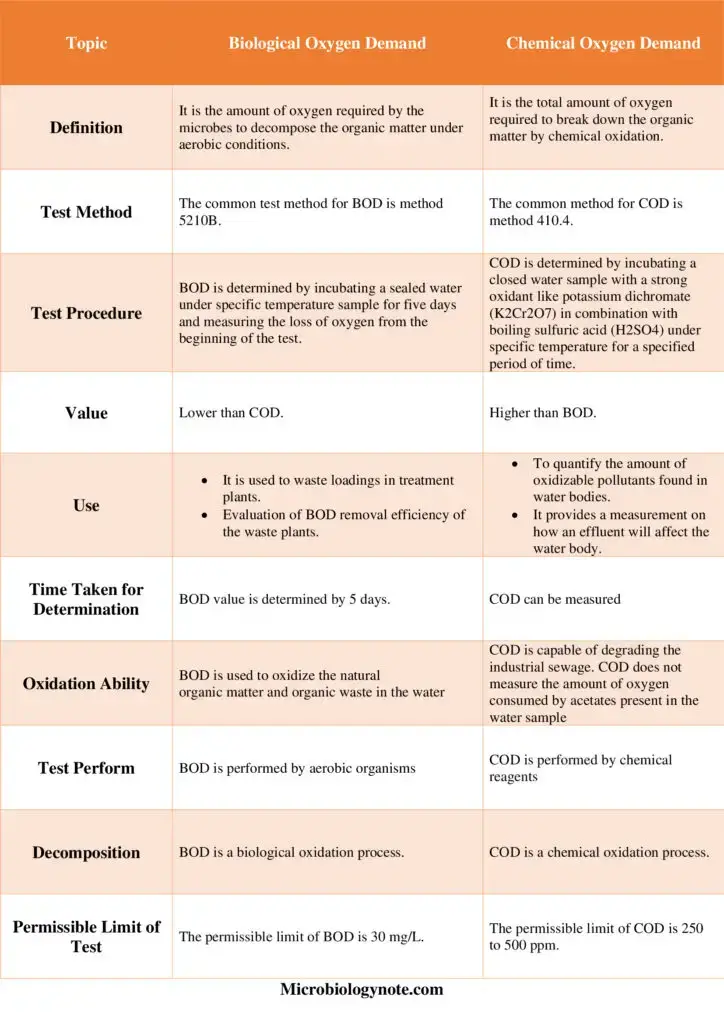
Similarities Between BOD and COD
- COD and BOD values can be expressed in mg/L or ppm (parts per million).
- Both values quantify the oxygen required to oxidise contaminants in water.
- Additionally, these readings show the degree of water pollution.
- In addition, both BOD and COD are essential in calculating the amount of trash in wastewater.
Difference between Biochemical Oxygen Demand (BOD) and Chemical Oxygen Demand (COD) pdf Download
References
- https://theconstructor.org/environmental-engg/difference-chemical-oxygen-demand-cod-biological-oxygen-demand-bod/34792/
- https://www.researchgate.net/profile/Lakna-Panawala/publication/318305894_Difference_Between_BOD_and_COD/links/5b08d33c4585157f87167337/Difference-Between-BOD-and-COD.pdf
- https://www.researchgate.net/profile/Lakna-Panawala/publication/318305894_Difference_Between_BOD_and_COD/links/5b08d33c4585157f87167337/Difference-Between-BOD-and-COD.pdf
- https://www.netsolwater.com/biological-oxygen-demand-vs-chemical-oxygen-demand.php?blog=1276
- https://www.differencebetween.com/difference-between-bod-and-vs-cod/
- https://camblab.info/what-are-the-differences-between-chemical-oxygen-demand-cod-and-biological-oxygen-demand-bod/
- https://butlerms.com/faq-items/what-is-the-difference-between-cod-and-bod/