What is Cytodifferentiation?
- Cytodifferentiation is a complex developmental process wherein cells undergo significant changes to acquire specialized structures and functions. This process plays a crucial role in plant tissue culture, particularly during the growth and maturation of callus tissue or free cells in suspension cultures. As these cells proliferate, some of them revert to a dedifferentiated state, leading to phenomena known as cytoquiescence and cytosenescence.
- Cytoquiescence refers to a state of temporary cell dormancy, while cytosenescence denotes the gradual deterioration of cellular functions over time. These two phenomena are critical for the differentiation of vascular tissues, especially in forming tracheary elements and sieve tubes, which are essential for the transport of water, nutrients, and sugars in plants.
- During cytodifferentiation, newly formed cells exhibit modifications that enhance their capacity to perform specific functions, often accompanied by distinct morphological changes. This specialization enables the cells to fulfill the requirements of various tissues in the plant, contributing to overall plant development and functionality.
Steps in Cytodifferentiation
Cytodifferentiation involves a series of critical steps that lead to the specialization of cells within a culture, particularly in plant tissue culture. The fate of individual cells during this process is inherently variable and influenced by both intrinsic and extrinsic factors. As a result, the cytodifferentiation process is not defined by a single event but is characterized by a dynamic interplay of conditions.
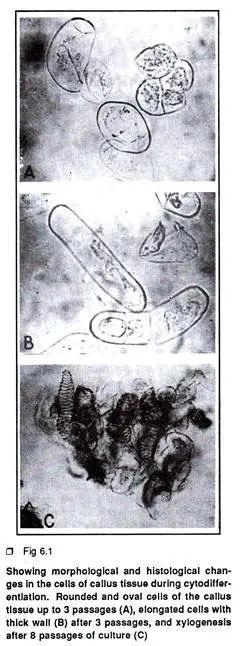
- Initial Cell Population: The primary steps in cytodifferentiation begin with the observation of callus tissue or free cells in suspension culture. Initially, this tissue exhibits a heterogeneous cell population comprising small, rounded, oval cells alongside a few elongated cells with dense cytoplasm.
- Morphogenetic Competence: Among these cells, only a select few develop morphogenetic competence for cytodifferentiation. This ability cannot be anticipated at the early stages of culture. The cells may either undergo spontaneous differentiation or respond to specific nutritional or hormonal stimuli.
- Histological and Biochemical Changes: As cytodifferentiation progresses, a series of histological and biochemical alterations occur. These changes can be categorized into three main steps. The first step involves repeated mitotic growth, where certain cells become elongated and develop thicker cell walls, leading to a friable callus tissue.
- Xylogenesis: As the number of subcultures increases, the callus tissue exhibits enhanced xylogenesis, characterized by the formation of tracheary elements that display a continuous spiral deposition of secondary wall materials. This xylogenesis primarily initiates from mitotically blocked and elongated cells.
- Intracellular Degradative Changes: The cytodifferentiation process is also marked by intracellular degradative changes. Notably, there is an autodestruction of cellular organelles, including chloroplasts, endoplasmic reticulum, dictyosomes, ribosomes, and mitochondria, which ultimately results in the loss of the entire protoplasmic mass.
- Cytoquiescence and Cytosenescence: The initial separation of the membranes surrounding these organelles represents the first step in cytoquiescence, which then leads to cytosenescence. This transition is intricately linked to the activity of certain hydrolytic enzymes, most notably acid phosphatase. This enzyme is commonly found within the cell, often associated with the cell wall, dictyosomes, plastids, and lysosomal systems, playing a pivotal role in cellular degradation.
- Role of Acid Phosphatase: The synthesis of acid phosphatase serves as an indicator of the autolysis of the protoplast during the states of cytoquiescence and cytosenescence. Therefore, the transformation of living cells into non-functional, empty tracheids during cellular differentiation is closely related to both the autolysis of cellular contents and the biosynthesis of lignin, which is essential for the formation of the secondary wall materials in developing tracheary elements.
Protocol for the Study of Cytodifferentiation
The study of cytodifferentiation involves examining the processes and changes that occur in plant cells as they differentiate into specialized structures, such as xylem elements. While there is no singular methodology for conducting this research, a well-established protocol can be utilized to observe the differentiation of xylem elements in the callus tissue of specific plants, such as Cowpea (Vigna unguiculata). This protocol includes several critical steps, outlined as follows:
- Selection of Initial Explant: The process begins with selecting the hypocotyl portion of aseptically grown seedlings of Vigna unguiculata as the initial explant. This choice is essential, as it provides a suitable source of cells that are likely to differentiate.
- Preparation of Culture Medium: The explant is then cultured in Murashige and Skoog’s medium, a widely used plant tissue culture medium. This medium is supplemented with 2,4-D (2-4 mg/L) and kinetin (0.5 mg/L). The presence of these hormones promotes cell division and differentiation. The culture is maintained at 25°C under conditions of 16 hours of light, facilitating optimal growth.
- Subculture Maintenance: Cultures are subjected to serial subculturing every 28 days. This step is crucial to ensure continuous growth and to promote the differentiation of the callus tissue over time.
- Harvesting and Maceration: Periodically, the callus tissue is harvested and macerated in a 4% aqueous solution of sodium hydroxide (NaOH) at 50°C. This treatment is designed to clear and soften the tissue, making it more amenable for subsequent staining and microscopic observation.
- Staining Process: Following maceration, the NaOH solution is replaced with a 0.04% aqueous solution of safranin. This dye stains the lignified structures, allowing for better visualization of differentiated cells. After 30 minutes of staining, the dye solution is replaced with 1N hydrochloric acid (HCl) at 50°C.
- Destaining and Slide Preparation: After one hour, the HCl is removed, and glycerol is added to the preparation. The acid destains the parenchyma cells, while the lignified xylem retains the red dye from the safranin, highlighting the differentiated structures.
- Microscopic Observation: Finally, a slide is prepared for microscopic examination. Observing the stained sections under a microscope allows researchers to analyze the differentiated xylem elements, providing insights into the processes of cytodifferentiation.
- https://www.biologydiscussion.com/plant-tissues/cytodifferentiation/cytodifferentiation-meaning-primary-steps-protocol-and-conclusion-plant-tissue-culture/14651
- https://plantcelltechnology.com/blogs/blog/blog-cytodifferentiation-and-tissue-culture?srsltid=AfmBOorbNtXsYcUBD17PIKgVWoAPwz5hE3YtaUfnmG72MkoRoxvpKUri
- https://www.slideshare.net/slideshow/cytodifferentiation-238401196/238401196
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC156987/pdf/091147.pdf